Visceral fat-specific regulation of plasminogen activator inhibitor-1 in aged septic mice
- PMID: 34369600
- PMCID: PMC8810697
- DOI: 10.1002/jcp.30551
Visceral fat-specific regulation of plasminogen activator inhibitor-1 in aged septic mice
Abstract
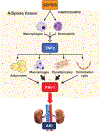
Elevated plasma levels of plasminogen activator inhibitor-1 (PAI-1) are documented in patients with sepsis and levels positively correlate with disease severity and mortality. Our previous work demonstrated that visceral adipose tissues (VAT) are a major source of PAI-1, especially in the aged (murine endotoxemia), that circulating PAI-1 protein levels match the trajectory of PAI-1 transcript levels in VAT (clinical sepsis), and that PAI-1 in both VAT and plasma are positively associated with acute kidney injury (AKI) in septic patients. In the current study utilizing preclinical sepsis models, PAI-1 tissue distribution was examined and cellular sources, as well as mechanisms mediating PAI-1 induction in VAT, were identified. In aged mice with sepsis, PAI-1 gene expression was significantly higher in VAT than in other major organs. VAT PAI-1 gene expression correlated with PAI-1 protein levels in both VAT and plasma. Moreover, VAT and plasma levels of PAI-1 were positively associated with AKI markers, modeling our previous clinical data. Using explant cultures of VAT, we determined that PAI-1 is secreted robustly in response to recombinant transforming growth factor β (TGFβ) and tumor necrosis factor α (TNFα) treatment; however, neutralization was effective only for TNFα indicating that TGFβ is not an endogenous modulator of PAI-1. Within VAT, TNFα was localized to neutrophils and macrophages. PAI-1 protein levels were fourfold higher in stromal vascular fraction (SVF) cells compared with mature adipocytes, and among SVF cells, both immune and nonimmune compartments expressed PAI-1 in a similar fashion. PAI-1 was localized predominantly to macrophages within the immune compartment and preadipocytes and endothelial cells within the nonimmune compartment. Collectively, these results indicate that induction and secretion of PAI-1 from VAT is facilitated by a complex interaction among immune and nonimmune cells. As circulating PAI-1 contributes to AKI in sepsis, understanding PAI-1 regulation in VAT could yield novel strategies for reducing systemic consequences of PAI-1 overproduction.
Keywords: PAI-1; adipose tissue; aging; kidney injury; sepsis.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Figures


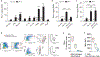
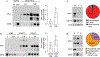
Similar articles
-
Adipose-Derived Inflammatory and Coagulant Mediators in Patients With Sepsis.Shock. 2021 May 1;55(5):596-606. doi: 10.1097/SHK.0000000000001579. Shock. 2021. PMID: 32496420 Free PMC article.
-
Abrogation of plasminogen activator inhibitor-1-vitronectin interaction ameliorates acute kidney injury in murine endotoxemia.PLoS One. 2015 Mar 23;10(3):e0120728. doi: 10.1371/journal.pone.0120728. eCollection 2015. PLoS One. 2015. PMID: 25799354 Free PMC article.
-
Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity.Diabetes. 2000 Aug;49(8):1374-80. doi: 10.2337/diabetes.49.8.1374. Diabetes. 2000. PMID: 10923640
-
The contribution of different adipose tissue depots to plasma plasminogen activator inhibitor-1 (PAI-1) levels.Blood Rev. 2016 Nov;30(6):421-429. doi: 10.1016/j.blre.2016.05.002. Epub 2016 May 19. Blood Rev. 2016. PMID: 27233154 Review.
-
Fat cell function and fibrinolysis.Horm Metab Res. 2000 Nov-Dec;32(11-12):504-8. doi: 10.1055/s-2007-978677. Horm Metab Res. 2000. PMID: 11246816 Review.
Cited by
-
PAI-1 as a critical factor in the resolution of sepsis and acute kidney injury in old age.Front Cell Dev Biol. 2024 Jan 18;11:1330433. doi: 10.3389/fcell.2023.1330433. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38304613 Free PMC article.
-
Effect of endothelial responses on sepsis-associated organ dysfunction.Chin Med J (Engl). 2024 Dec 5;137(23):2782-2792. doi: 10.1097/CM9.0000000000003342. Epub 2024 Nov 6. Chin Med J (Engl). 2024. PMID: 39501810 Free PMC article. Review.
-
The Impact of Biological Sex And High-Fat High-Fructose Diet on Brain Dysfunction in an Animal Model of Sepsis.Mol Neurobiol. 2025 Apr 23. doi: 10.1007/s12035-025-04937-y. Online ahead of print. Mol Neurobiol. 2025. PMID: 40268828
-
Risk for Cardiovascular Death Associated With Waist Circumference and Diabetes: A 9-Year Prospective Study in the Wan Shou Lu Cohort.Front Cardiovasc Med. 2022 Apr 26;9:856517. doi: 10.3389/fcvm.2022.856517. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35557528 Free PMC article.
-
Accumulation of γδ T cells in visceral fat with aging promotes chronic inflammation.Geroscience. 2022 Jun;44(3):1761-1778. doi: 10.1007/s11357-022-00572-w. Epub 2022 Apr 28. Geroscience. 2022. PMID: 35477832 Free PMC article.
References
-
- Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M,…Juhan-Vague I (2000). Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes, 49(8), 1374–1380. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, & Pinsky MR (2001). Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med, 29(7), 1303–1310. - PubMed
-
- Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H,…Bellomo R (2010). Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med, 36(3), 452–461. - PubMed
-
- Chen R, Yan J, Liu P, Wang Z, & Wang C (2017). Plasminogen activator inhibitor links obesity and thrombotic cerebrovascular diseases: The roles of PAI-1 and obesity on stroke. Metab Brain Dis, 32(3), 667–673. - PubMed
-
- Chronopoulos A, Rosner MH, Cruz DN, & Ronco C (2010). Acute kidney injury in elderly intensive care patients: a review. Intensive Care Med, 36(9), 1454–1464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

