Understanding the Interactions Between the Ocular Surface Microbiome and the Tear Proteome
- PMID: 34369983
- PMCID: PMC8354087
- DOI: 10.1167/iovs.62.10.8
Understanding the Interactions Between the Ocular Surface Microbiome and the Tear Proteome
Abstract
Purpose: The purpose of this study was to explore the interplay between the ocular surface microbiome and the tear proteome in humans in order to better understand the pathogenesis of ocular surface-associated diseases.
Methods: Twenty eyes from 20 participants were included in the study. The ocular surface microbiome was sequenced by whole-metagenome shotgun sequencing using lid and conjunctival swabs. Furthermore, the tear proteome was identified using chromatography tandem mass spectrometry. After compositional and functional profiling of the metagenome and functional characterization of the proteome by gene ontology, association studies between the ocular microbiome and tear proteome were assessed.
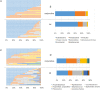
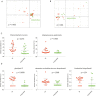
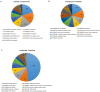
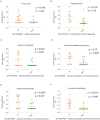
Results: Two hundred twenty-nine taxa were identified with Actinobacteria and Proteobacteria being the most abundant phyla with significantly more Propionibacterium acnes and Staphylococcus epidermidis in lid compared to conjunctival swabs. The lid metagenomes were enriched in genes of the glycolysis lll and adenosine nucleotides de novo and L-isoleucine biosynthesis. Correlations between the phylum Firmicutes and fatty acid metabolism, between the genus Agrobacterium as well as vitamin B1 synthesis and antimicrobial activity, and between biosynthesis of heme, L-arginine, as well as L-citrulline and human vision were detected.
Conclusions: The ocular surface microbiome was found to be associated with the tear proteome with a role in human immune defense. This study has a potential impact on the development of treatment strategies for ocular surface-associated diseases.
Trial registration: ClinicalTrials.gov NCT04656197.
Conflict of interest statement
Disclosure:
Figures