A novel neurotensin/xenin fusion peptide enhances β-cell function and exhibits antidiabetic efficacy in high-fat fed mice
- PMID: 34370015
- PMCID: PMC8390788
- DOI: 10.1042/BSR20211275
A novel neurotensin/xenin fusion peptide enhances β-cell function and exhibits antidiabetic efficacy in high-fat fed mice
Abstract
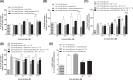
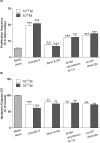
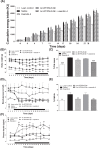
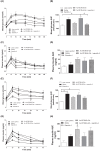
Neurotensin and xenin possess antidiabetic potential, mediated in part through augmentation of incretin hormone, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), action. In the present study, fragment peptides of neurotensin and xenin, acetyl-neurotensin and xenin-8-Gln, were fused together to create Ac-NT/XN-8-Gln. Following assessment of enzymatic stability, effects of Ac-NT/XN-8-Gln on in vitro β-cell function were studied. Subchronic antidiabetic efficacy of Ac-NT/XN-8-Gln alone, and in combination with the clinically approved GLP-1 receptor agonist exendin-4, was assessed in high-fat fed (HFF) mice. Ac-NT/XN-8-Gln was highly resistant to plasma enzyme degradation and induced dose-dependent insulin-releasing actions (P<0.05 to P<0.01) in BRIN-BD11 β-cells and isolated mouse islets. Ac-NT/XN-8-Gln augmented (P<0.001) the insulinotropic actions of GIP, while possessing independent β-cell proliferative (P<0.001) and anti-apoptotic (P<0.01) actions. Twice daily treatment of HFF mice with Ac-NT/XN-8-Gln for 32 days improved glycaemic control and circulating insulin, with benefits significantly enhanced by combined exendin-4 treatment. This was reflected by reduced body fat mass (P<0.001), improved circulating lipid profile (P<0.01) and reduced HbA1c concentrations (P<0.01) in the combined treatment group. Following an oral glucose challenge, glucose levels were markedly decreased (P<0.05) only in combination treatment group and superior to exendin-4 alone, with similar observations made in response to glucose plus GIP injection. The combined treatment group also presented with improved insulin sensitivity, decreased pancreatic insulin content as well as increased islet and β-cell areas. These data reveal that Ac-NT/XN-8-Gln is a biologically active neurotensin/xenin fusion peptide that displays prominent antidiabetic efficacy when administered together with exendin-4.
Keywords: Neurotensin; Xenin; hybrid; incretin.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
A novel GLP-1/xenin hybrid peptide improves glucose homeostasis, circulating lipids and restores GIP sensitivity in high fat fed mice.Peptides. 2018 Feb;100:202-211. doi: 10.1016/j.peptides.2017.10.015. Peptides. 2018. PMID: 29412820
-
Antidiabetic effects and sustained metabolic benefits of sub-chronic co-administration of exendin-4/gastrin and xenin-8-Gln in high fat fed mice.Eur J Pharmacol. 2019 Dec 15;865:172733. doi: 10.1016/j.ejphar.2019.172733. Epub 2019 Oct 12. Eur J Pharmacol. 2019. PMID: 31614140
-
A GIP/xenin hybrid in combination with exendin-4 improves metabolic status in db/db diabetic mice and promotes enduring antidiabetic benefits in high fat fed mice.Biochem Pharmacol. 2020 Jan;171:113723. doi: 10.1016/j.bcp.2019.113723. Epub 2019 Nov 20. Biochem Pharmacol. 2020. PMID: 31756326
-
beta-cell failure in diabetes and preservation by clinical treatment.Endocr Rev. 2007 Apr;28(2):187-218. doi: 10.1210/10.1210/er.2006-0038. Epub 2007 Mar 12. Endocr Rev. 2007. PMID: 17353295 Review.
-
Emerging therapeutic potential for xenin and related peptides in obesity and diabetes.Diabetes Metab Res Rev. 2018 Sep;34(6):e3006. doi: 10.1002/dmrr.3006. Epub 2018 Apr 26. Diabetes Metab Res Rev. 2018. PMID: 29633491 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

