A phase 1, open-label, drug-drug interaction study of rucaparib with rosuvastatin and oral contraceptives in patients with advanced solid tumors
- PMID: 34370076
- PMCID: PMC8484168
- DOI: 10.1007/s00280-021-04338-7
A phase 1, open-label, drug-drug interaction study of rucaparib with rosuvastatin and oral contraceptives in patients with advanced solid tumors
Abstract
Purpose: This study aimed at evaluating the effect of rucaparib on the pharmacokinetics of rosuvastatin and oral contraceptives in patients with advanced solid tumors and the safety of rucaparib with and without coadministration of rosuvastatin or oral contraceptives.
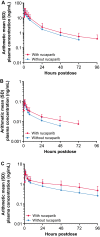
Methods: Patients received single doses of oral rosuvastatin 20 mg (Arm A) or oral contraceptives ethinylestradiol 30 µg + levonorgestrel 150 µg (Arm B) on days 1 and 19 and continuous doses of rucaparib 600 mg BID from day 5 to 23. Serial blood samples were collected with and without rucaparib for pharmacokinetic analysis.
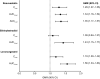
Results: Thirty-six patients (n = 18 each arm) were enrolled and received at least 1 dose of study drug. In the drug-drug interaction analysis (n = 15 each arm), the geometric mean ratio (GMR) of maximum concentration (Cmax) with and without rucaparib was 1.29 for rosuvastatin, 1.09 for ethinylestradiol, and 1.19 for levonorgestrel. GMR of area under the concentration-time curve from time zero to last quantifiable measurement (AUC0-last) was 1.34 for rosuvastatin, 1.43 for ethinylestradiol, and 1.56 for levonorgestrel. There was no increase in frequency of treatment-emergent adverse events (TEAEs) when rucaparib was given with either of the probe drugs. In both arms, most TEAEs were mild in severity and considered unrelated to study treatment.
Conclusion: Rucaparib 600 mg BID weakly increased the plasma exposure to rosuvastatin or oral contraceptives. Rucaparib safety profile when coadministered with rosuvastatin or oral contraceptives was consistent with that of rucaparib monotherapy. Dose adjustments of rosuvastatin and oral contraceptives are not necessary when coadministered with rucaparib. ClinicalTrials.gov NCT03954366; Date of registration May 17, 2019.
Keywords: BCRP; Drug–drug interaction; Oncology; Oral contraceptives; Rucaparib.
© 2021. The Author(s).
Conflict of interest statement
RR has served in a consulting or advisory role for Pfizer, Takeda, Boehringer Ingelheim, Roche, MSD, Novartis, and Bristol-Myers Squibb. EN has served in a consulting or advisory role for Bay Trials Unlimited, LLC. ML, JL, SE, JH, NG, and JX are employees of Clovis Oncology and may own stock or have stock options in that company. KGJ, MT-K, IL, MJ, VS, PC, and MG declare no conflict of interest.
Figures

References
-
- Robillard L, Nguyen M, Harding TC, Simmons AD. In vitro and in vivo assessment of the mechanism of action of the PARP inhibitor rucaparib. Cancer Res. 2017 doi: 10.1158/1538-7445.AM2017-2475. - DOI
-
- Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, Mukhopadhyay A, Los G, Hostomsky Z, Plummer ER, Edmondson RJ, Curtin NJ. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011;103(4):334–346. doi: 10.1093/jnci/djq509. - DOI - PubMed
-
- Rubraca (rucaparib) tablets [prescribing information] (2020). Clovis Oncology, Inc., Boulder
-
- Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, Domchek SM, Balmana J, Drew Y, Chen LM, Safra T, Montes A, Giordano H, Maloney L, Goble S, Isaacson J, Xiao J, Borrow J, Rolfe L, Shapira-Frommer R. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–4106. doi: 10.1158/1078-0432.CCR-16-2796. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

