Analysis of the Manganese and MntR Regulon in Corynebacterium diphtheriae
- PMID: 34370555
- PMCID: PMC8459757
- DOI: 10.1128/JB.00274-21
Analysis of the Manganese and MntR Regulon in Corynebacterium diphtheriae
Abstract
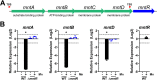
Corynebacterium diphtheriae is the causative agent of a severe respiratory disease in humans. The bacterial systems required for infection are poorly understood, but the acquisition of metals such as manganese (Mn) is likely critical for host colonization. MntR is an Mn-dependent transcriptional regulator in C. diphtheriae that represses the expression of the mntABCD genes, which encode a putative ABC metal transporter. However, other targets of Mn and MntR regulation in C. diphtheriae have not been identified. In this study, we use comparisons between the gene expression profiles of wild-type C. diphtheriae strain 1737 grown without or with Mn supplementation and comparisons of gene expression between the wild type and an mntR deletion mutant to characterize the C. diphtheriae Mn and MntR regulon. MntR was observed to both repress and induce various target genes in an Mn-dependent manner. Genes induced by MntR include the Mn-superoxide dismutase, sodA, and the putative ABC transporter locus, iutABCD. DNA binding studies showed that MntR interacts with the promoter regions for several genes identified in the expression study, and a 17-bp consensus MntR DNA binding site was identified. We found that an mntR mutant displayed increased sensitivity to Mn and cadmium that could be alleviated by the additional deletion of the mntABCD transport locus, providing evidence that the MntABCD transporter functions as an Mn uptake system in C. diphtheriae. The findings in this study further our understanding of metal uptake systems and global metal regulatory networks in this important human pathogen. IMPORTANCE Mechanisms for metal scavenging are critical to the survival and success of bacterial pathogens, including Corynebacterium diphtheriae. Metal import systems in pathogenic bacteria have been studied as possible vaccine components due to high conservation, critical functionality, and surface localization. In this study, we expand our understanding of the genes controlled by the global manganese regulator, MntR. We determined a role for the MntABCD transporter in manganese import using evidence from manganese and cadmium toxicity assays. Understanding the nutritional requirements of C. diphtheriae and the tools used to acquire essential metals will aid in the development of future vaccines.
Keywords: Corynebacterium; diphtheria; manganese.
Figures
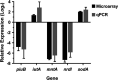




References
-
- Attia AS, Schroeder KA, Seeley EH, Wilson KJ, Hammer ND, Colvin DC, Manier ML, Nicklay JJ, Rose KL, Gore JC, Caprioli RM, Skaar EP. 2012. Monitoring the inflammatory response to infection through the integration of MALDI IMS and MRI. Cell Host Microbe 11:664–673. 10.1016/j.chom.2012.04.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

