Obesity protects against sepsis-induced and norepinephrine-induced white adipose tissue browning
- PMID: 34370596
- PMCID: PMC8461795
- DOI: 10.1152/ajpendo.00380.2020
Obesity protects against sepsis-induced and norepinephrine-induced white adipose tissue browning
Abstract
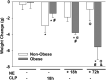
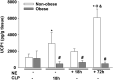
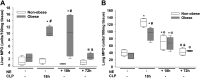
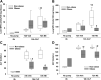
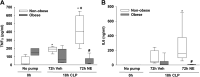
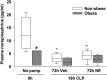
Sepsis is a dysregulated systemic response to infection and can lead to organ damage and death. Obesity is a significant problem worldwide and affects outcomes from sepsis. Our laboratory demonstrated that white adipose tissue (WAT) undergoes browning during sepsis, a process whereby WAT adopts a brown adipose tissue phenotype. However, this browning process was not observed in obese mice during sepsis. White adipose tissue browning is detrimental in patients with burn injury and cancer. We hypothesize that norepinephrine (NE) induces WAT browning in nonobese mice but not in obese mice similarly to sepsis-induced WAT browning. Six-week-old C57BL/6 male mice were randomized to a high-fat diet or normal diet. After 6-7 wk of feeding, polymicrobial sepsis was induced by cecal ligation and puncture (CLP). Norepinephrine was administered intraperitoneally via osmotic minipumps for 18 h or 72 h (no CLP) at which time tissue and plasma were harvested. Controls were mice that underwent CLP (no NE) with 18-h harvest. A separate group of mice underwent pretreatment with NE or vehicle infusion for 72 h, CLP was performed, and at 18 h had tissue and plasma harvested. Sepsis resulted in significant weight loss in both nonobese and obese mice. NE treatment alone caused weight loss in obese mice. Septic nonobese mice had higher uncoupling protein-1 (UCP1) expression compared with control and obese septic mice. NE treatment increased UCP1 expression in nonobese, but not obese mice. NE-treated obese septic mice had lower lung myeloperoxidase (MPO) activity, alanine aminotransferase (ALT), aspartate aminotransferase (AST), TNFα, and IL-6 levels compared with NE-treated nonobese septic mice. Obesity protects mice from septic-induced and NE-induced WAT browning.NEW & NOTEWORTHY White adipose tissue browning is detrimental in patients with burn injury and cancer. WAT browning occurs in nonobese mice and can be induced by β receptor norepinephrine infusion, but obese mice are resistant to sepsis-induced and norepinephrine-induced WAT browning. We propose that the lack of WAT browning and unchanged inflammatory cytokine response may contribute to the protection of obese mice from sepsis.
Keywords: adipose tissue; browning; obesity; sepsis.
Conflict of interest statement
J.K. reports financial support to the institution for work on DSMB of a clinical trial (Eli Lilly) and participation on a sepsis advisory board (Roche) which are not related in any way to the work in the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

