Modern Acinetobacter baumannii clinical isolates replicate inside spacious vacuoles and egress from macrophages
- PMID: 34370792
- PMCID: PMC8376066
- DOI: 10.1371/journal.ppat.1009802
Modern Acinetobacter baumannii clinical isolates replicate inside spacious vacuoles and egress from macrophages
Abstract
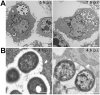
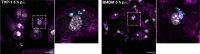
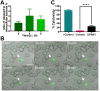
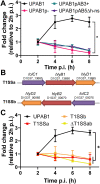
Multidrug-resistant Acinetobacter baumannii infections are increasing at alarming rates. Therefore, novel antibiotic-sparing treatments to combat these A. baumannii infections are urgently needed. The development of these interventions would benefit from a better understanding of this bacterium's pathobiology, which remains poorly understood. A. baumannii is regarded as an extracellular opportunistic pathogen. However, research on Acinetobacter has largely focused on common lab strains, such as ATCC 19606, that have been isolated several decades ago. These strains exhibit reduced virulence when compared to recently isolated clinical strains. In this work, we demonstrate that, unlike ATCC 19606, several modern A. baumannii clinical isolates, including the recent clinical urinary isolate UPAB1, persist and replicate inside macrophages within spacious vacuoles. We show that intracellular replication of UPAB1 is dependent on a functional type I secretion system (T1SS) and pAB5, a large conjugative plasmid that controls the expression of several chromosomally-encoded genes. Finally, we show that UPAB1 escapes from the infected macrophages by a lytic process. To our knowledge, this is the first report of intracellular growth and replication of A. baumannii. We suggest that intracellular replication within macrophages may contribute to evasion of the immune response, dissemination, and antibiotic tolerance of A. baumannii.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al.. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37: 1288–1301. doi: 10.1017/ice.2016.174 - DOI - PMC - PubMed
-
- Tacconelli E, Magrini N, Kahlmeter G, Singh N. Global Priority List of Antibiotic-Resistant Bacteria To Guide Research, Discovery, and Development of New Antibiotics. 2017.
-
- Centers for Disease Control U. Antibiotic Resistance Threats in the United States, 2019. [cited 8 Apr 2021]. doi: 10.15620/cdc:82532 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

