Anticancer drug-induced life-threatening ventricular arrhythmias: a World Health Organization pharmacovigilance study
- PMID: 34370839
- PMCID: PMC8677441
- DOI: 10.1093/eurheartj/ehab362
Anticancer drug-induced life-threatening ventricular arrhythmias: a World Health Organization pharmacovigilance study
Abstract
Aims: With the explosion of anticancer drugs, an emerging concern is the risk for drug-induced sudden death (SD) via ventricular arrhythmias (VA).
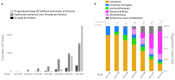
Methods and results: We used the international pharmacovigilance database VigiBase (n = 18 441 659 reports) to compare drug-induced long QT (diLQT, n = 18 123) and VA (n = 29 193) including torsade de pointes (TdP, n = 8163) reporting for 663 anticancer drugs vs. all other drugs until 01/01/2019. The analysis used the 95% lower-end credibility interval of the information component (IC025), an indicator for disproportionate Bayesian reporting; significant when IC025 >0. There were 2301 reports (13.8% fatal) for 40 anticancer drugs significantly associated with diLQT (with 27 also associated with VA or SD) and 9 drugs associated with VA without diLQT. Half of these (46.9%, 23/49) were associated with SD. Most (41%, 20/49) were kinase inhibitors, 8% (4/49) were hormonal therapies, 6% (3/49) were immunotherapies, 24% (12/49) were cytotoxics, and 20% (10/49) were miscellaneous. In VigiBase, reports of diLQT, TdP, or VA increased from 580 in the period 1967-83 to 15 070 in 2014-18 with the proportion related to anticancer drugs increasing from 0.9% (5/580) to 14.0% (2115/15 070) (P < 0.0001). Concordance between these VigiBase signals and data concerning diLQT and VA/TdP identified in CredibleMeds or US Food and Drug Administration (FDA) labels was moderate (κ = 0.47 and 0.40, P < 0.0001). Twenty-three drugs represent new signals, while 24 flagged by CredibleMeds or FDA had no signal in VigiBase. A three-level SD risk stratification relying on isolated long QT (low risk), associated with VA without SD (moderate risk), and VA with SD (high risk) is proposed.
Conclusion: This list of liable anticancer drugs may prove useful for physicians and regulatory authorities to re-evaluate cardiac monitoring requirements.
Clinical trial registration: NCT03530215.
Keywords: Anticancer drugs; Disproportionality analysis; Long QT; Pharmacovigilance; Torsade de pointes; Ventricular arrhythmias.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
QT prolongation and cancer therapeutics: a coming Tempest or Much Ado About Nothing?Eur Heart J. 2021 Oct 7;42(38):3929-3931. doi: 10.1093/eurheartj/ehab483. Eur Heart J. 2021. PMID: 34387663 No abstract available.
References
-
- Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust T, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019;20:1493–1505. - PMC - PubMed
-
- Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. - PubMed
-
- Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017;18:e445–e456. - PubMed
-
- Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E, Funck-Brentano E, Moslehi JJ, Johnson DB, Salem JE. Clinical pharmacology and interplay of immune checkpoint agents: a Yin-Yang balance. Annu Rev Pharmacol Toxicol 2021;61:85–112. - PubMed
-
- Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano J-P, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. - PMC - PubMed

