Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial
- PMID: 34370971
- PMCID: PMC8346248
- DOI: 10.1016/S0140-6736(21)01694-9
Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial
Abstract
Background: Use of heterologous prime-boost COVID-19 vaccine schedules could facilitate mass COVID-19 immunisation. However, we have previously reported that heterologous schedules incorporating an adenoviral vectored vaccine (ChAdOx1 nCoV-19, AstraZeneca; hereafter referred to as ChAd) and an mRNA vaccine (BNT162b2, Pfizer-BioNTech; hereafter referred to as BNT) at a 4-week interval are more reactogenic than homologous schedules. Here, we report the safety and immunogenicity of heterologous schedules with the ChAd and BNT vaccines.
Methods: Com-COV is a participant-blinded, randomised, non-inferiority trial evaluating vaccine safety, reactogenicity, and immunogenicity. Adults aged 50 years and older with no or well controlled comorbidities and no previous SARS-CoV-2 infection by laboratory confirmation were eligible and were recruited at eight sites across the UK. The majority of eligible participants were enrolled into the general cohort (28-day or 84-day prime-boost intervals), who were randomly assigned (1:1:1:1:1:1:1:1) to receive ChAd/ChAd, ChAd/BNT, BNT/BNT, or BNT/ChAd, administered at either 28-day or 84-day prime-boost intervals. A small subset of eligible participants (n=100) were enrolled into an immunology cohort, who had additional blood tests to evaluate immune responses; these participants were randomly assigned (1:1:1:1) to the four schedules (28-day interval only). Participants were masked to the vaccine received but not to the prime-boost interval. The primary endpoint was the geometric mean ratio (GMR) of serum SARS-CoV-2 anti-spike IgG concentration (measured by ELISA) at 28 days after boost, when comparing ChAd/BNT with ChAd/ChAd, and BNT/ChAd with BNT/BNT. The heterologous schedules were considered non-inferior to the approved homologous schedules if the lower limit of the one-sided 97·5% CI of the GMR of these comparisons was greater than 0·63. The primary analysis was done in the per-protocol population, who were seronegative at baseline. Safety analyses were done among participants receiving at least one dose of a study vaccine. The trial is registered with ISRCTN, 69254139.
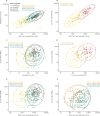
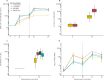
Findings: Between Feb 11 and Feb 26, 2021, 830 participants were enrolled and randomised, including 463 participants with a 28-day prime-boost interval, for whom results are reported here. The mean age of participants was 57·8 years (SD 4·7), with 212 (46%) female participants and 117 (25%) from ethnic minorities. At day 28 post boost, the geometric mean concentration of SARS-CoV-2 anti-spike IgG in ChAd/BNT recipients (12 906 ELU/mL) was non-inferior to that in ChAd/ChAd recipients (1392 ELU/mL), with a GMR of 9·2 (one-sided 97·5% CI 7·5 to ∞). In participants primed with BNT, we did not show non-inferiority of the heterologous schedule (BNT/ChAd, 7133 ELU/mL) against the homologous schedule (BNT/BNT, 14 080 ELU/mL), with a GMR of 0·51 (one-sided 97·5% CI 0·43 to ∞). Four serious adverse events occurred across all groups, none of which were considered to be related to immunisation.
Interpretation: Despite the BNT/ChAd regimen not meeting non-inferiority criteria, the SARS-CoV-2 anti-spike IgG concentrations of both heterologous schedules were higher than that of a licensed vaccine schedule (ChAd/ChAd) with proven efficacy against COVID-19 disease and hospitalisation. Along with the higher immunogenicity of ChAd/BNT compared with ChAD/ChAd, these data support flexibility in the use of heterologous prime-boost vaccination using ChAd and BNT COVID-19 vaccines.
Funding: UK Vaccine Task Force and National Institute for Health Research.
Crown Copyright © 2021 Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
Declaration of interests MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM. He receives no personal financial payment for this work. JSN-V-T is seconded to the Department of Health and Social Care, England. AMC and DMF are investigators on studies funded by Pfizer and Unilever. They receive no personal financial payment for this work. AF is a member of the Joint Committee on Vaccination and Immunisation and chair of the WHO European Technical Advisory Group of Experts on Immunisation. He is an investigator or provides consultative advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia. He receives no personal remuneration or benefits for any of this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator or provides consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva. He receives no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, and Valneva. He receives no personal financial payment for this work. CAG acts on behalf of University Hospitals Birmingham NHS Foundation Trust as an investigator on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, CureVac, Moderna, and Valneva. He receives no personal financial payment for this work. VL acts on behalf of University College London Hospitals NHS Foundation Trust as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers including Pfizer, AstraZeneca, and Valneva. He receives no personal financial payment for this work. TL is named as an inventor on a patent application covering the ChAd vaccine and is an occasional consultant to Vaccitech, unrelated to this work. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. All other authors declare no competing interests.
Figures


Comment in
-
Optimising SARS-CoV-2 vaccination schedules.Lancet. 2021 Sep 4;398(10303):819-821. doi: 10.1016/S0140-6736(21)01729-3. Epub 2021 Aug 6. Lancet. 2021. PMID: 34370969 Free PMC article. No abstract available.
References
-
- WHO Impact of COVID-19 on people's livelihoods, their health and our food systems. Oct 13, 2020. https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people%27...
-
- WHO Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. 2021. https://extranet.who.int/pqweb/key-resources/documents/status-covid-19-v...
-
- WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int
-
- Our World in Data Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations
-
- COVAX Workshop report: COVAX clinical development & operations SWAT team workshop on “Booster and mix & match COVID-19 vaccine strategies—planning ahead in an environment of increasing complexity”. June 3, 2021. https://media.tghn.org/medialibrary/2021/07/20210603_COVAX_Booster_Mix_M...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

