Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study
- PMID: 34370972
- PMCID: PMC8346241
- DOI: 10.1016/S0140-6736(21)01608-1
Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study
Abstract
Background: A new syndrome of vaccine-induced immune thrombotic thrombocytopenia (VITT) has emerged as a rare side-effect of vaccination against COVID-19. Cerebral venous thrombosis is the most common manifestation of this syndrome but, to our knowledge, has not previously been described in detail. We aimed to document the features of post-vaccination cerebral venous thrombosis with and without VITT and to assess whether VITT is associated with poorer outcomes.
Methods: For this multicentre cohort study, clinicians were asked to submit all cases in which COVID-19 vaccination preceded the onset of cerebral venous thrombosis, regardless of the type of vaccine, interval between vaccine and onset of cerebral venous thrombosis symptoms, or blood test results. We collected clinical characteristics, laboratory results (including the results of tests for anti-platelet factor 4 antibodies where available), and radiological features at hospital admission of patients with cerebral venous thrombosis after vaccination against COVID-19, with no exclusion criteria. We defined cerebral venous thrombosis cases as VITT-associated if the lowest platelet count recorded during admission was below 150 × 109 per L and, if the D-dimer was measured, the highest value recorded was greater than 2000 μg/L. We compared the VITT and non-VITT groups for the proportion of patients who had died or were dependent on others to help them with their activities of daily living (modified Rankin score 3-6) at the end of hospital admission (the primary outcome of the study). The VITT group were also compared with a large cohort of patients with cerebral venous thrombosis described in the International Study on Cerebral Vein and Dural Sinus Thrombosis.
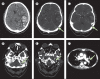
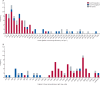
Findings: Between April 1 and May 20, 2021, we received data on 99 patients from collaborators in 43 hospitals across the UK. Four patients were excluded because they did not have definitive evidence of cerebral venous thrombosis on imaging. Of the remaining 95 patients, 70 had VITT and 25 did not. The median age of the VITT group (47 years, IQR 32-55) was lower than in the non-VITT group (57 years; 41-62; p=0·0045). Patients with VITT-associated cerebral venous thrombosis had more intracranial veins thrombosed (median three, IQR 2-4) than non-VITT patients (two, 2-3; p=0·041) and more frequently had extracranial thrombosis (31 [44%] of 70 patients) compared with non-VITT patients (one [4%] of 25 patients; p=0·0003). The primary outcome of death or dependency occurred more frequently in patients with VITT-associated cerebral venous thrombosis (33 [47%] of 70 patients) compared with the non-VITT control group (four [16%] of 25 patients; p=0·0061). This adverse outcome was less frequent in patients with VITT who received non-heparin anticoagulants (18 [36%] of 50 patients) compared with those who did not (15 [75%] of 20 patients; p=0·0031), and in those who received intravenous immunoglobulin (22 [40%] of 55 patients) compared with those who did not (11 [73%] of 15 patients; p=0·022).
Interpretation: Cerebral venous thrombosis is more severe in the context of VITT. Non-heparin anticoagulants and immunoglobulin treatment might improve outcomes of VITT-associated cerebral venous thrombosis. Since existing criteria excluded some patients with otherwise typical VITT-associated cerebral venous thrombosis, we propose new diagnostic criteria that are more appropriate.
Funding: None.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests RJP receives grants from Randox Laboratories on an unrelated subject and from The Stroke Association for work on COVID-19 and stroke, not related to vaccination. PA-F receives grants from the Wellcome Trust for work on an unrelated subject. EH receives grants from MND Scotland and the National Institute for Health Research (NIHR) for work on an unrelated subject. TS sits on the Medicines and Healthcare products Regulatory Agency Vaccine Benefit Versus Risk Expert Working Group and was on the Data Safety Monitoring Committee of the GSK study to evaluate the safety and immunogenicity of a candidate Ebola vaccine in children GSK3390107A (ChAd3 EBO-Z) vaccine. MS receives grants from Shire and Novartis, and has received personal fees from Takeda, Novartis, Octapharma, and Sanofi for work on unrelated subjects. BS received a grant from the Medical Research Council, via the UK Research Institutes/NIHR Global Effort on COVID-19 Research to study neurological disease in relation to COVID-19, and has been a case management consultant to WHO-South-East Asia via the Global Outbreak Alert and Response Network since April, 2020, but vaccination against the infection is not the focus in either case. CR receives grants from the NIHR for work on an unrelated subject and is also collaborating with FirstKind Medical on a grant on an unrelated subject. CR is chair of the NIHR Hyperacute Stroke Research Oversight Group and is a member of the European Stroke Organization board of directors. DJW has received personal fees from Bayer, Alnylam, and Portola, unrelated to the work presented here. All other authors declare no competing interests.
Figures

Comment in
-
Cerebral venous sinus thrombosis after vaccination: the UK experience.Lancet. 2021 Sep 25;398(10306):1107-1109. doi: 10.1016/S0140-6736(21)01788-8. Epub 2021 Aug 6. Lancet. 2021. PMID: 34370974 Free PMC article. No abstract available.
References
-
- Our World in Data Coronavirus (COVID-19) deaths. https://ourworldindata.org/covid-deaths
-
- Our World in Data Coronarivus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

