Ethanol modulation of cortico-basolateral amygdala circuits: Neurophysiology and behavior
- PMID: 34371080
- PMCID: PMC8478846
- DOI: 10.1016/j.neuropharm.2021.108750
Ethanol modulation of cortico-basolateral amygdala circuits: Neurophysiology and behavior
Abstract
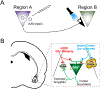
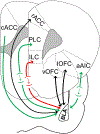
This review highlights literature relating the anatomy, physiology, and behavioral contributions by projections between rodent prefrontal cortical areas and the basolateral amygdala. These projections are robustly modulated by both environmental experience and exposure to drugs of abuse including ethanol. Recent literature relating optogenetic and chemogenetic dissection of these circuits within behavior both compliments and occasionally challenges roles defined by more traditional pharmacological or lesion-based approaches. In particular, cortico-amygdala circuits help control both aversive and reward-seeking. Exposure to pathology-producing environments or abused drugs dysregulates the relative 'balance' of these outcomes. Modern circuit-based approaches have also shown that overlapping populations of neurons within a given brain region frequently govern both aversion and reward-seeking. In addition, these circuits often dramatically influence 'local' cortical or basolateral amygdala excitatory or inhibitory circuits. Our understanding of these neurobiological processes, particularly in relation to ethanol research, has just begun and represents a significant opportunity. This article is part of the special Issue on 'Neurocircuitry Modulating Drug and Alcohol Abuse'.
Keywords: Abused drugs; Chemogenetics; Conditioned behavior; Optogenetics.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
References
-
- Alexander GM, Graef JD, Hammarback JA, Nordskog BK, Burnett EJ, Daunais JB, Bennett AJ, Friedman DP, Suomi SJ, Godwin DW, 2012. Disruptions in serotonergic regulation of cortical glutamate release in primate insular cortex in response to chronic ethanol and nursery rearing. Neuroscience 207, 167–81. 10.1016/j.neuroscience.2012.01.027 - DOI - PMC - PubMed
-
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, Ba D, Brown EN, Tye KM, 2018. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342. 10.1016/j.cell.2018.04.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

