Clinical benefit of systemic therapies for recurrent ovarian cancer-ESMO-MCBS scores
- PMID: 34371383
- PMCID: PMC8358417
- DOI: 10.1016/j.esmoop.2021.100229
Clinical benefit of systemic therapies for recurrent ovarian cancer-ESMO-MCBS scores
Abstract
Background: Licensed systemic treatment options for platinum-sensitive recurrent ovarian cancer are platinum-based chemotherapy and maintenance treatment with bevacizumab and poly (ADP-ribose) polymerase inhibitors. For platinum-resistant disease, several non-platinum options are available. We aimed to assess the clinical benefit of these treatments according to the European Society of Medical Oncology (ESMO)-Magnitude of Clinical Benefit Scale (MCBS).
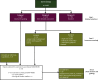
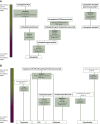
Materials and methods: A PubMed search was carried out including all studies evaluating systemic treatment of recurrent epithelial ovarian cancer, from 1990 onwards. Randomised trials with an adequate comparator and design showing a statistically significant benefit of the study arm were independently scored by two blinded observers using the ESMO-MCBS.
Results: A total of 1127 papers were identified, out of which 61 reported results of randomised trials of sufficient quality. Nineteen trials showed statistically significant results and the studied treatments were graded according to ESMO-MCBS. Only three treatments showed substantial benefit (score of 4 on a scale of 1-5) according to the ESMO-MCBS: platinum-based chemotherapy with paclitaxel in the platinum-sensitive setting and the addition of bevacizumab to chemotherapy in the platinum-resistant setting. The WEE1 inhibitor adavosertib (not licensed) also scores a 4, based on a recent small phase II study. Assessment of quality-of-life data and toxicity using the ESMO-MCBS showed to be complex, which should be taken into account in using this score for clinical decision making.
Conclusion: Only a few licensed systemic therapies for recurrent ovarian cancer show substantial clinical benefit based on ESMO-MCBS scores. Trials demonstrating overall survival benefit are sparse.
Keywords: ESMO-MCBS; chemotherapy; clinical benefit; ovarian cancer; targeted therapy.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure MJ serves on the advisory board of AstraZeneca and Merck, with funding to institution. The remaining authors have declared no conflicts of interest.
Figures
References
-
- Colombo N., Sessa C., du Bois A. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. - PubMed
-
- ESMO Guidelines Committee eUpdate 2020—Relapsed epithelial ovarian carcinoma treatment recommendations. https://www.esmo.org/guidelines/gynaecological-cancers/newly-diagnosed-a... Available at. Accessed March 22, 2021.
-
- Cherny N.I., Dafni U., Bogaerts J. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28:2340–2366. - PubMed
-
- Pignata S., Scambia G., Bologna A. Randomized controlled trial testing the efficacy of platinum-free interval prolongation in advanced ovarian cancer: the MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG study. J Clin Oncol. 2017;35:3347–3353. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

