How DNA damage and non-canonical nucleotides alter the telomerase catalytic cycle
- PMID: 34371388
- PMCID: PMC8526386
- DOI: 10.1016/j.dnarep.2021.103198
How DNA damage and non-canonical nucleotides alter the telomerase catalytic cycle
Abstract
Telomeres at the ends of linear chromosomes are essential for genome maintenance and sustained cellular proliferation, but shorten with each cell division. Telomerase, a specialized reverse transcriptase with its own integral RNA template, compensates for this by lengthening the telomeric 3' single strand overhang. Mammalian telomerase has the unique ability to processively synthesize multiple GGTTAG repeats, by translocating along its product and reiteratively copying the RNA template, termed repeat addition processivity (RAP). This unusual form of processivity is distinct from the nucleotide addition processivity (NAP) shared by all other DNA polymerases. In this review, we focus on the minimally active human telomerase catalytic core consisting of the telomerase reverse transcriptase (TERT) and the integral RNA (TR), which catalyzes DNA synthesis. We review the mechanisms by which oxidatively damaged nucleotides, and anti-viral and anti-cancer nucleotide drugs affect the telomerase catalytic cycle. Finally, we offer perspective on how we can leverage telomerase's unique properties, and advancements in understanding of telomerase catalytic mechanism, to selectively manipulate telomerase activity with therapeutics, particularly in cancer treatment.
Keywords: Aging; Cancer; DNA damage; DNA repair; Nucleoside analogs; Ribonucleotides; Telomerase; Telomerase therapeutics; Telomeres.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest
Figures
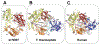


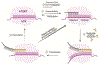

Similar articles
-
A self-regulating template in human telomerase.Proc Natl Acad Sci U S A. 2014 Aug 5;111(31):11311-6. doi: 10.1073/pnas.1402531111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982163 Free PMC article.
-
Structures of telomerase at several steps of telomere repeat synthesis.Nature. 2021 May;593(7859):454-459. doi: 10.1038/s41586-021-03529-9. Epub 2021 May 12. Nature. 2021. PMID: 33981033 Free PMC article.
-
DNA-binding determinants and cellular thresholds for human telomerase repeat addition processivity.EMBO J. 2017 Jul 3;36(13):1908-1927. doi: 10.15252/embj.201796887. Epub 2017 May 11. EMBO J. 2017. PMID: 28495680 Free PMC article.
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
Telomeres and telomerase.Philos Trans R Soc Lond B Biol Sci. 2004 Jan 29;359(1441):109-21. doi: 10.1098/rstb.2003.1370. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15065663 Free PMC article. Review.
Cited by
-
Mechanisms of telomere maintenance and associated therapeutic vulnerabilities in malignant gliomas.Neuro Oncol. 2024 Jun 3;26(6):1012-1024. doi: 10.1093/neuonc/noae016. Neuro Oncol. 2024. PMID: 38285162 Free PMC article. Review.
-
Recent advancements in the structural biology of human telomerase and their implications for improved design of cancer therapeutics.NAR Cancer. 2023 Mar 3;5(1):zcad010. doi: 10.1093/narcan/zcad010. eCollection 2023 Mar. NAR Cancer. 2023. PMID: 36879683 Free PMC article.
-
Chemotherapeutic 6-thio-2'-deoxyguanosine selectively targets and inhibits telomerase by inducing a non-productive telomere-bound telomerase complex.bioRxiv [Preprint]. 2025 Jun 27:2025.02.05.636339. doi: 10.1101/2025.02.05.636339. bioRxiv. 2025. PMID: 39975053 Free PMC article. Preprint.
-
Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress.Front Cell Dev Biol. 2021 Nov 19;9:758402. doi: 10.3389/fcell.2021.758402. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869348 Free PMC article. Review.
-
Mitochondria and telomeres: hand in glove.Biogerontology. 2024 Apr;25(2):289-300. doi: 10.1007/s10522-023-10074-7. Epub 2023 Oct 21. Biogerontology. 2024. PMID: 37864609 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

