Therapeutic Development Based on the Immunopathogenic Mechanisms of Psoriasis
- PMID: 34371756
- PMCID: PMC8308930
- DOI: 10.3390/pharmaceutics13071064
Therapeutic Development Based on the Immunopathogenic Mechanisms of Psoriasis
Abstract
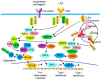
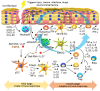
Psoriasis, a complex inflammatory autoimmune skin disorder that affects 2-3% of the global population, is thought to be genetically predetermined and induced by environmental and immunological factors. In the past decades, basic and clinical studies have significantly expanded knowledge on the molecular, cellular, and immunological mechanisms underlying the pathogenesis of psoriasis. Based on these pathogenic mechanisms, the current disease model emphasizes the role of aberrant Th1 and Th17 responses. Th1 and Th17 immune responses are regulated by a complex network of different cytokines, including TNF-α, IL-17, and IL-23; signal transduction pathways downstream to the cytokine receptors; and various activated transcription factors, including NF-κB, interferon regulatory factors (IRFs), and signal transducer and activator of transcriptions (STATs). The biologics developed to specifically target the cytokines have achieved a better efficacy and safety for the systemic management of psoriasis compared with traditional treatments. Nevertheless, the current therapeutics can only alleviate the symptoms; there is still no cure for psoriasis. Therefore, the development of more effective, safe, and affordable therapeutics for psoriasis is important. In this review, we discussed the current trend of therapeutic development for psoriasis based on the recent discoveries in the immune modulation of the inflammatory response in psoriasis.
Keywords: Toll-like receptor; anti-psoriasis drugs; autoimmune; skin inflammation; therapeutic antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
- 110L901402B to L.-C.H./Ministry of Education in Taiwan, National Taiwan University
- 110-EC-17-A-22-1700 to T.-H.C./Ministry of Economic Affairs of Taiwan
- 108-2320-B-002-020-MY3 and 110-2634-F-002-044 to L.-C.H., 107-2320-B-400 -016 -MY3 to T.-H.C./Ministry of Science and Technology (MOST) of Taiwan
- NHRI-EX110-11031SI to L.-C.H., IM-110-PP-02 to T.-H.C./National Health Research Institutes, Taiwan
- 108L893604 to L.-C.H./National Taiwan University
LinkOut - more resources
Full Text Sources
Miscellaneous

