Endoplasmic Reticulum Stress and Autophagy Markers in Soleus Muscle Disuse-Induced Atrophy of Rats Treated with Fish Oil
- PMID: 34371808
- PMCID: PMC8308346
- DOI: 10.3390/nu13072298
Endoplasmic Reticulum Stress and Autophagy Markers in Soleus Muscle Disuse-Induced Atrophy of Rats Treated with Fish Oil
Abstract
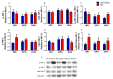
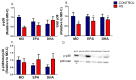
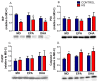
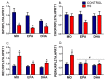
Endoplasmic reticulum stress (ERS) and autophagy pathways are implicated in disuse muscle atrophy. The effects of high eicosapentaenoic (EPA) or high docosahexaenoic (DHA) fish oils on soleus muscle ERS and autophagy markers were investigated in a rat hindlimb suspension (HS) atrophy model. Adult Wistar male rats received daily by gavage supplementation (0.3 mL per 100 g b.w.) of mineral oil or high EPA or high DHA fish oils (FOs) for two weeks. Afterward, the rats were subjected to HS and the respective treatments concomitantly for an additional two-week period. After four weeks, we evaluated ERS and autophagy markers in the soleus muscle. Results were analyzed using two-way analysis of variance (ANOVA) and Bonferroni post hoc test. Gastrocnemius muscle ω-6/ω-3 fatty acids (FAs) ratio was decreased by both FOs indicating the tissue incorporation of omega-3 fatty acids. HS altered (p < 0.05) the protein content (decreasing total p38 and BiP and increasing p-JNK2/total JNK2 ratio, and caspase 3) and gene expressions (decreasing BiP and increasing IRE1 and PERK) of ERS and autophagy (decreasing Beclin and increasing LC3 and ATG14) markers in soleus. Both FOs attenuated (p < 0.05) the increase in PERK and ATG14 expressions induced by HS. Thus, both FOs could potentially attenuate ERS and autophagy in skeletal muscles undergoing atrophy.
Keywords: docosahexaenoic acid; eicosapentanoic acid; hindlimb suspension; skeletal muscle atrophy; unfolded protein response; ω-3 fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Attenuation of diabetes-mediated muscle atrophy in rats by fish oil enriched omega-3 polyunsaturated fatty acids supplementation.J Food Drug Anal. 2023 Aug 31;31(3):458-472. doi: 10.38212/2224-6614.3468. J Food Drug Anal. 2023. PMID: 39666277 Free PMC article.
-
Effects of high EPA and high DHA fish oils on changes in signaling associated with protein metabolism induced by hindlimb suspension in rats.Physiol Rep. 2016 Sep;4(18):e12958. doi: 10.14814/phy2.12958. Physiol Rep. 2016. PMID: 27650250 Free PMC article.
-
Skeletal muscle atrophy induced by aging and disuse atrophy are strongly associated with the upregulation of the endoplasmic stress protein CHOP in rats.Mol Biol Rep. 2025 Mar 18;52(1):322. doi: 10.1007/s11033-025-10415-4. Mol Biol Rep. 2025. PMID: 40100290 Free PMC article.
-
Endoplasmic reticulum stress and unfolded protein response: Roles in skeletal muscle atrophy.Biochem Pharmacol. 2025 Apr;234:116799. doi: 10.1016/j.bcp.2025.116799. Epub 2025 Feb 12. Biochem Pharmacol. 2025. PMID: 39952329 Review.
-
Omega-3 fatty acids and human skeletal muscle.Curr Opin Clin Nutr Metab Care. 2021 Mar 1;24(2):114-119. doi: 10.1097/MCO.0000000000000723. Curr Opin Clin Nutr Metab Care. 2021. PMID: 33332930 Review.
Cited by
-
Advances in nutritional supplementation for sarcopenia management.Front Nutr. 2023 Jul 10;10:1189522. doi: 10.3389/fnut.2023.1189522. eCollection 2023. Front Nutr. 2023. PMID: 37492597 Free PMC article. Review.
-
New Horizons in the Treatment of Age-Associated Obesity, Sarcopenia and Osteoporosis.Drugs Aging. 2022 Sep;39(9):673-683. doi: 10.1007/s40266-022-00960-z. Epub 2022 Jul 4. Drugs Aging. 2022. PMID: 35781216
-
Attenuation of diabetes-mediated muscle atrophy in rats by fish oil enriched omega-3 polyunsaturated fatty acids supplementation.J Food Drug Anal. 2023 Aug 31;31(3):458-472. doi: 10.38212/2224-6614.3468. J Food Drug Anal. 2023. PMID: 39666277 Free PMC article.
-
Low-frequency electrical stimulation alleviates immobilization-evoked disuse muscle atrophy by repressing autophagy in skeletal muscle of rabbits.BMC Musculoskelet Disord. 2022 Apr 28;23(1):398. doi: 10.1186/s12891-022-05350-5. BMC Musculoskelet Disord. 2022. PMID: 35484550 Free PMC article.
References
-
- De Vasconcelos D.A.A., Giesbertz P., de Souza D.R., Vitzel K.F., Abreu P., Marzuca-Nassr G.N., Fortes M.A.S., Murata G.M., Hirabara S.M., Curi R., et al. Oral L-glutamine pretreatment attenuates skeletal muscle atrophy induced by 24-h fasting in mice. J. Nutr. Biochem. 2019;70:202–214. doi: 10.1016/j.jnutbio.2019.05.010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

