Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model
- PMID: 34371879
- PMCID: PMC8308809
- DOI: 10.3390/nu13072366
Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model
Abstract
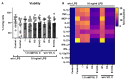
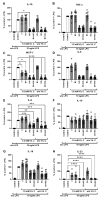
Sepsis is an extremely complex clinical syndrome, usually involving an excessive inflammatory response including an overshooting cytokine release that damages tissue and organs of the patient. Due to the severity of this condition, it is estimated that over 11 million people die from sepsis each year. Despite intensive research in the field, there is still no specific therapy for sepsis. Many sepsis patients show a marked deficiency of vitamin C. 9 out of 10 sepsis patients have a hypovitaminosis C, and every third patient even shows a clinical deficiency in the scurvy range. In addition, low vitamin C levels of intensive care sepsis patients correlate with a higher need for vasopressors, higher Sequential Organ Failure Assessment (SOFA) scores, and increased mortality. Based on this observation and the conducted clinical trials using vitamin C as sepsis therapy in intensive care patients, the aim of the present ex vivo study was to evaluate the effects of high-dose vitamin C alone and in a triple combination supplemented with vitamin B1 (thiamine) and hydrocortisone on the lipopolysaccharide (LPS)-induced cytokine response in peripheral blood mononuclear cells (PBMCs) from healthy human donors. We found that all corticosteroid combinations strongly reduced the cytokine response on RNA- and protein levels, while high-dose vitamin C alone significantly diminished the PBMC mediated secretion of the cytokines interleukin (IL)-10, IL-23, and monocyte chemo-attractant protein (MCP-1), which mediate the inflammatory response. However, vitamin C showed no enhancing effect on the secretion of further cytokines studied. This data provides important insights into the possible immunomodulatory function of vitamin C in an ex vivo setting of human PBMCs and the modulation of their cytokine profile in the context of sepsis. Since vitamin C is a vital micronutrient, the restoration of physiologically adequate concentrations should be integrated into routine sepsis therapy, and the therapeutic effects of supraphysiological concentrations of vitamin C in sepsis patients should be further investigated in clinical trials.
Keywords: cytokine release; hydrocortisone; sepsis; vitamin B1; vitamin C.
Conflict of interest statement
A.L., M.B., H.N., C.L., O.R., C.B., T.S. and S.V. declare that they have no competing interests. C.V. and H.M., as employees of Pascoe pharmazeutische Praeparate GmbH (Giessen, Germany), received the ready designed manuscript and were not involved in the content-related structuring. Furthermore, C.V. and H.M. had no influence on the acquisition and interpretation of the data or literature used.
Figures



References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Vincent J.L., de Mendonça A., Cantraine F., Moreno R., Takala J., Suter P.M., Sprung C.L., Colardyn F., Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

