Both Isocarbohydrate and Hypercarbohydrate Fruit Preloads Curbed Postprandial Glycemic Excursion in Healthy Subjects
- PMID: 34371978
- PMCID: PMC8308803
- DOI: 10.3390/nu13072470
Both Isocarbohydrate and Hypercarbohydrate Fruit Preloads Curbed Postprandial Glycemic Excursion in Healthy Subjects
Abstract
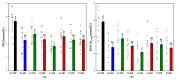
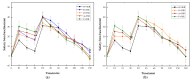
This study aimed to investigate the impact of fruit preloads on the acute postprandial glycemic response (PGR) and satiety response of a rice meal in healthy female subjects based on iso-carbohydrate (IC) and hyper-carbohydrate (HC) contents, respectively. The IC test meals including (1) rice preload (R + 35R), (2) orange preload (O + 35R), (3) apple preload (A + 35R) and (4) pear preload (P + 35R), contained 50.0 g available carbohydrates (AC) where the preload contributed 15.0 g and rice provided 35.0 g. The HC meals included (1) orange preload (O + 50R), (2) apple preload (A+50R) and (3) pear preload (P + 50R), each containing 65.0 g AC, where the fruits contributed 15.0 g and rice provided 50.0 g. Drinking water 30 min before the rice meal was taken as reference (W + 50R). All the preload treatments, irrespective of IC or HC meals, resulted in remarkable reduction (p < 0.001) in terms of incremental peak glucose (IPG) and the maximum amplitude of glycemic excursion in 180 min (MAGE0-180), also a significant decrease (p < 0.05) in the area of PGR contributed by per gram of AC (AAC), compared with the W + 50R. Apple elicited the lowest PGR among all test meals, as the A + 35R halved the IPG and slashed the incremental area under the curve in 180 min (iAUC0-180) by 45.7%, while the A + 50R reduced the IPG by 29.7%, compared with the W + 50R. All the preload meals and the reference meal showed comparable self-reported satiety in spite of the difference in AC. In conclusion, pre-meal consumption of three fruits effectively curbed post-meal glycemia even in the case of a 30% extra carbohydrate load.
Keywords: apple; fruit; glycemic response; preload; satiety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yang C.-H., Chang C.-W., Lin J. White Rice Glycemic Index Measured in Venous and Capillary Blood Samples. Food Sci. Technol. Res. 2017;23:297–304. doi: 10.3136/fstr.23.297. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

