Advances in Functionalized Photosensitive Polymeric Nanocarriers
- PMID: 34372067
- PMCID: PMC8348146
- DOI: 10.3390/polym13152464
Advances in Functionalized Photosensitive Polymeric Nanocarriers
Abstract
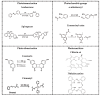
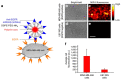
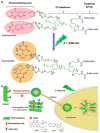
The synthesis of light-responsive nanocarriers (LRNs) with a variety of surface functional groups and/or ligands has been intensively explored for space-temporal controlled cargo release. LRNs have been designed on demand for photodynamic-, photothermal-, chemo-, and radiotherapy, protected delivery of bioactive molecules, such as smart drug delivery systems and for theranostic duties. LRNs trigger the release of cargo by a light stimulus. The idea of modifying LRNs with different moieties and ligands search for site-specific cargo delivery imparting stealth effects and/or eliciting specific cellular interactions to improve the nanosystems' safety and efficacy. This work reviews photoresponsive polymeric nanocarriers and photo-stimulation mechanisms, surface chemistry to link ligands and characterization of the resultant nanosystems. It summarizes the interesting biomedical applications of functionalized photo-controlled nanocarriers, highlighting the current challenges and opportunities of such high-performance photo-triggered delivery systems.
Keywords: functional nanoparticle; light-responsive drug delivery; photoresponsive nanoparticle; polymer nanocarrier.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gai S., Yang G., Yang P., He F., Lin J., Jin D., Xing B. Recent Advances in Functional Nanomaterials for Light–Triggered Cancer Therapy. Nano Today. 2018;19:146–187. doi: 10.1016/j.nantod.2018.02.010. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

