Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior
- PMID: 34372179
- PMCID: PMC8348702
- DOI: 10.3390/polym13152577
Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior
Abstract
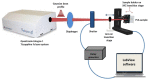
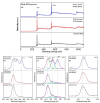
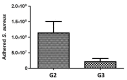
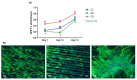
The influence of ultra-short laser modification on the surface morphology and possible chemical alteration of poly-lactic acid (PLA) matrix in respect to the optimization of cellular and antibacterial behavior were investigated in this study. Scanning electron microscopy (SEM) morphological examination of the processed PLA surface showed the formation of diverse hierarchical surface microstructures, generated by irradiation with a range of laser fluences (F) and scanning velocities (V) values. By controlling the laser parameters, diverse surface roughness can be achieved, thus influencing cellular dynamics. This surface feedback can be applied to finely tune and control diverse biomaterial surface properties like wettability, reflectivity, and biomimetics. The triggering of thermal effects, leading to the ejection of material with subsequent solidification and formation of raised rims and 3D-like hollow structures along the processed zones, demonstrated a direct correlation to the wettability of the PLA. A transition from superhydrophobic (θ > 150°) to super hydrophilic (θ < 20°) surfaces can be achieved by the creation of grooves with V = 0.6 mm/s, F = 1.7 J/cm2. The achieved hierarchical architecture affected morphology and thickness of the processed samples which were linked to the nature of ultra-short laser-material interaction effects, namely the precipitation of temperature distribution during material processing can be strongly minimized with ultrashort pulses leading to non-thermal and spatially localized effects that can facilitate volume ablation without collateral thermal damage The obtained modification zones were analyzed employing Fourier transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS), Energy dispersive X-ray analysis (EDX), and optical profilometer. The modification of the PLA surface resulted in an increased roughness value for treatment with lower velocities (V = 0.6 mm/s). Thus, the substrate gains a 3D-like architecture and forms a natural matrix by microprocessing with V = 0.6 mm/s, F = 1.7 J/cm2, and V = 3.8 mm/s, F = 0.8 J/cm2. The tests performed with Mesenchymal stem cells (MSCs) demonstrated that the ultra-short laser surface modification altered the cell orientation and promoted cell growth. The topographical design was tested also for the effectiveness of bacterial attachment concerning chosen parameters for the creation of an array with defined geometrical patterns.
Keywords: Fs bioscaffolds structuring; PLA texturing; cell matrices; temporal scaffolds; tissue engineering; ultra-short functionalization of cell matrices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- World Population Prospects: The 2019 Revision. United Nations DESA/Population Division. 2019. [(accessed on 26 March 2021)]; Available online: https://population.un.org/wpp/
Grants and funding
LinkOut - more resources
Full Text Sources

