Fabrication and Characterization of Iridium Oxide pH Microelectrodes Based on Sputter Deposition Method
- PMID: 34372233
- PMCID: PMC8348779
- DOI: 10.3390/s21154996
Fabrication and Characterization of Iridium Oxide pH Microelectrodes Based on Sputter Deposition Method
Abstract
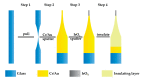
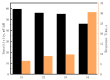
pH value plays an important role in many fields such as chemistry and biology; therefore, rapid and accurate pH measurement is very important. Because of its advantages in preparation, wide test range, rapid response, and good biocompatibility, iridium oxide material has received more and more attention. In this paper, we present a method for preparing iridium oxide pH microelectrodes based on the sputter deposition method. The sputtering parameters of iridium oxide are also studied and optimized. Open-circuit potential tests show that microelectrodes exhibit near-Nernstian pH response with good linearity (about 60 mV/pH), fast response, high stability (a slight periodic fluctuation of potential change <2.5 mV in 24 h), and good reversibility in the pH range of 1.00-13.00.
Keywords: iridium oxide; long-term stability; near-Nernstian response; pH electrode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zhou Z., Li J., Pan D., Wei H., Wang C., Pan F., Xia J., Ma S. pH electrodes based on iridium oxide films for marine monitoring. Trends Environ. Anal. Chem. 2020;25:e83. doi: 10.1016/j.teac.2020.e00083. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

