Multiplexed Prostate Cancer Companion Diagnostic Devices
- PMID: 34372259
- PMCID: PMC8347987
- DOI: 10.3390/s21155023
Multiplexed Prostate Cancer Companion Diagnostic Devices
Abstract
Prostate cancer (PCa) remains one of the most prominent forms of cancer for men. Since the early 1990s, Prostate-Specific Antigen (PSA) has been a commonly recognized PCa-associated protein biomarker. However, PSA testing has been shown to lack in specificity and sensitivity when needed to diagnose, monitor and/or treat PCa patients successfully. One enhancement could include the simultaneous detection of multiple PCa-associated protein biomarkers alongside PSA, also known as multiplexing. If conventional methods such as the enzyme-linked immunosorbent assay (ELISA) are used, multiplexed detection of such protein biomarkers can result in an increase in the required sample volume, in the complexity of the analytical procedures, and in adding to the cost. Using companion diagnostic devices such as biosensors, which can be portable and cost-effective with multiplexing capacities, may address these limitations. This review explores recent research for multiplexed PCa protein biomarker detection using optical and electrochemical biosensor platforms. Some of the novel and potential serum-based PCa protein biomarkers will be discussed in this review. In addition, this review discusses the importance of converting research protocols into multiplex point-of-care testing (xPOCT) devices to be used in near-patient settings, providing a more personalized approach to PCa patients' diagnostic, surveillance and treatment management.
Keywords: companion diagnostic devices; multiplex point-of-care testing (xPOCT); prostate cancer; protein biomarkers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



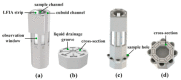
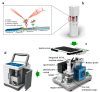

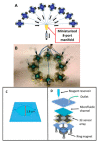
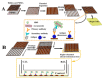
Similar articles
-
Simultaneous noninvasive ultrasensitive detection of prostate specific antigen and lncRNA PCA3 using multiplexed dual optical microfibers with strong plasmonic nanointerfaces.Biosens Bioelectron. 2024 Nov 15;264:116672. doi: 10.1016/j.bios.2024.116672. Epub 2024 Aug 14. Biosens Bioelectron. 2024. PMID: 39151263
-
Electrochemical prostate-specific antigen biosensors based on electroconductive nanomaterials and polymers.Clin Chim Acta. 2021 May;516:111-135. doi: 10.1016/j.cca.2021.01.018. Epub 2021 Feb 2. Clin Chim Acta. 2021. PMID: 33545110 Review.
-
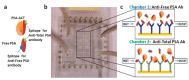
Multiplexed Opto-Microfluidic Biosensing: Advanced Platform for Prostate Cancer Detection.ACS Sens. 2024 May 24;9(5):2596-2604. doi: 10.1021/acssensors.4c00312. Epub 2024 Apr 29. ACS Sens. 2024. PMID: 38683677
-
Simultaneous and combined detection of multiple tumor biomarkers for prostate cancer in human serum by suspension array technology.Biosens Bioelectron. 2013 Sep 15;47:92-8. doi: 10.1016/j.bios.2013.02.052. Epub 2013 Mar 20. Biosens Bioelectron. 2013. PMID: 23567627
-
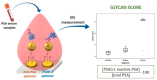
Glycosylated biomarker sensors: advancements in prostate cancer diagnosis.Chem Commun (Camb). 2021 Sep 23;57(76):9640-9655. doi: 10.1039/d1cc03080a. Chem Commun (Camb). 2021. PMID: 34473143 Review.
Cited by
-
Laser-assisted protein micropatterning in a thermoplastic device for multiplexed prostate cancer biomarker detection.Lab Chip. 2023 Jan 31;23(3):534-541. doi: 10.1039/d2lc00840h. Lab Chip. 2023. PMID: 36642981 Free PMC article.
-
Extracellular vesicles as a new frontier of diagnostic biomarkers in osteosarcoma diseases: a bibliometric and visualized study.Front Oncol. 2024 Mar 4;14:1359807. doi: 10.3389/fonc.2024.1359807. eCollection 2024. Front Oncol. 2024. PMID: 38500663 Free PMC article.
-
Exosomes as a new frontier of cancer liquid biopsy.Mol Cancer. 2022 Feb 18;21(1):56. doi: 10.1186/s12943-022-01509-9. Mol Cancer. 2022. PMID: 35180868 Free PMC article. Review.
-
Towards Multiplexed and Multimodal Biosensor Platforms in Real-Time Monitoring of Metabolic Disorders.Sensors (Basel). 2022 Jul 12;22(14):5200. doi: 10.3390/s22145200. Sensors (Basel). 2022. PMID: 35890880 Free PMC article. Review.
-
Translational Research for Identifying Potential Early-stage Prostate Cancer Biomarkers.Cancer Genomics Proteomics. 2023 Jan-Feb;20(1):1-8. doi: 10.21873/cgp.20359. Cancer Genomics Proteomics. 2023. PMID: 36581341 Free PMC article.
References
-
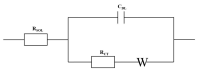
- Traynor S.M., Pandey R., Maclachlan R., Hosseini A., Didar T.F., Li F., Soleymani L. Review—Recent advances in electrochemical detection of prostate specific antigen (PSA) in clinically-relevant samples. J. Electrochem. Soc. 2020;167:037551. doi: 10.1149/1945-7111/ab69fd. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

