A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus
- PMID: 34372350
- PMCID: PMC8346961
- DOI: 10.3390/s21155114
A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus
Abstract
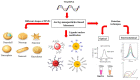
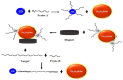
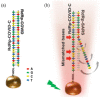
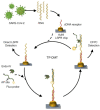
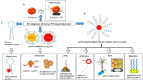
The emergence of highly pathogenic and deadly human coronaviruses, namely SARS-CoV and MERS-CoV within the past two decades and currently SARS-CoV-2, have resulted in millions of human death across the world. In addition, other human viral diseases, such as mosquito borne-viral diseases and blood-borne viruses, also contribute to a higher risk of death in severe cases. To date, there is no specific drug or medicine available to cure these human viral diseases. Therefore, the early and rapid detection without compromising the test accuracy is required in order to provide a suitable treatment for the containment of the diseases. Recently, nanomaterials-based biosensors have attracted enormous interest due to their biological activities and unique sensing properties, which enable the detection of analytes such as nucleic acid (DNA or RNA), aptamers, and proteins in clinical samples. In addition, the advances of nanotechnologies also enable the development of miniaturized detection systems for point-of-care (POC) biosensors, which could be a new strategy for detecting human viral diseases. The detection of virus-specific genes by using single-stranded DNA (ssDNA) probes has become a particular interest due to their higher sensitivity and specificity compared to immunological methods based on antibody or antigen for early diagnosis of viral infection. Hence, this review has been developed to provide an overview of the current development of nanoparticles-based biosensors that target pathogenic RNA viruses, toward a robust and effective detection strategy of the existing or newly emerging human viral diseases such as SARS-CoV-2. This review emphasizes the nanoparticles-based biosensors developed using noble metals such as gold (Au) and silver (Ag) by virtue of their powerful characteristics as a signal amplifier or enhancer in the detection of nucleic acid. In addition, this review provides a broad knowledge with respect to several analytical methods involved in the development of nanoparticles-based biosensors for the detection of viral nucleic acid using both optical and electrochemical techniques.
Keywords: biosensor; electrochemical biosensing; nanoparticles; nucleic acid; plasmonic; viral disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Development of gold nanoparticle-based biosensors for COVID-19 diagnosis.Beni Suef Univ J Basic Appl Sci. 2022;11(1):111. doi: 10.1186/s43088-022-00293-1. Epub 2022 Sep 5. Beni Suef Univ J Basic Appl Sci. 2022. PMID: 36092513 Free PMC article. Review.
-
AuNP-based biosensors for the diagnosis of pathogenic human coronaviruses: COVID-19 pandemic developments.Anal Bioanal Chem. 2022 Oct;414(24):7069-7084. doi: 10.1007/s00216-022-04193-2. Epub 2022 Jul 4. Anal Bioanal Chem. 2022. PMID: 35781591 Free PMC article. Review.
-
Nanoparticle electrochemical biosensors for virus detection.Clin Chim Acta. 2025 Jan 30;566:120054. doi: 10.1016/j.cca.2024.120054. Epub 2024 Nov 16. Clin Chim Acta. 2025. PMID: 39551230 Review.
-
Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor.Anal Chim Acta. 2021 Oct 16;1182:338939. doi: 10.1016/j.aca.2021.338939. Epub 2021 Aug 11. Anal Chim Acta. 2021. PMID: 34602210 Free PMC article.
-
Emerging Trends of Gold Nanostructures for Point-of-Care Biosensor-Based Detection of COVID-19.Mol Biotechnol. 2025 Apr;67(4):1398-1422. doi: 10.1007/s12033-024-01157-y. Epub 2024 May 4. Mol Biotechnol. 2025. PMID: 38703305 Review.
Cited by
-
Emerging Applications of Nanobiosensors in Pathogen Detection in Water and Food.Biosensors (Basel). 2023 Oct 11;13(10):922. doi: 10.3390/bios13100922. Biosensors (Basel). 2023. PMID: 37887115 Free PMC article. Review.
-
Impact of nanotechnology on conventional and artificial intelligence-based biosensing strategies for the detection of viruses.Discov Nano. 2023 Apr 1;18(1):58. doi: 10.1186/s11671-023-03842-4. eCollection 2023 Dec. Discov Nano. 2023. PMID: 37032711 Free PMC article. Review.
-
Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors.Sensors (Basel). 2022 Mar 7;22(5):2072. doi: 10.3390/s22052072. Sensors (Basel). 2022. PMID: 35271219 Free PMC article.
-
Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay.Front Bioeng Biotechnol. 2023 Jan 10;10:1090281. doi: 10.3389/fbioe.2022.1090281. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36704307 Free PMC article. Review.
-
Electrochemical biosensor detection on respiratory and flaviviruses.Appl Microbiol Biotechnol. 2023 Mar;107(5-6):1503-1513. doi: 10.1007/s00253-023-12400-y. Epub 2023 Jan 31. Appl Microbiol Biotechnol. 2023. PMID: 36719432 Free PMC article. Review.
References
-
- Peiris J.S.M., Poon L.L.M. Severe Acute Respiratory Syndrome (SARS) Encycl. Virol. 2008:552–556. doi: 10.1016/B978-012374410-4.00780-9. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

