Antivirals against the Chikungunya Virus
- PMID: 34372513
- PMCID: PMC8310245
- DOI: 10.3390/v13071307
Antivirals against the Chikungunya Virus
Abstract
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that has re-emerged in recent decades, causing large-scale epidemics in many parts of the world. CHIKV infection leads to a febrile disease known as chikungunya fever (CHIKF), which is characterised by severe joint pain and myalgia. As many patients develop a painful chronic stage and neither antiviral drugs nor vaccines are available, the development of a potent CHIKV inhibiting drug is crucial for CHIKF treatment. A comprehensive summary of current antiviral research and development of small-molecule inhibitor against CHIKV is presented in this review. We highlight different approaches used for the identification of such compounds and further discuss the identification and application of promising viral and host targets.
Keywords: Chikungunya virus; alphavirus; antiviral drug development; antiviral therapy; direct-acting antivirals; host-directed antivirals; in silico screening; in vivo validation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
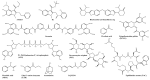



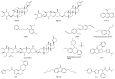

References
-
- Vazeille M., Moutailler S., Coudrier D., Rousseaux C., Khun H., Huerre M., Thiria J., Bastien Dehecq J.-S., Fontenille D., Schuffenecker I., et al. Two Chikungunya Isolates from Two Chikungunya Isolates from the Outbreak of La Reunion (Indian Ocean) Exhibit Different Patterns of Infection in the Mosquito, Aedes albopictus. PLoS ONE. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

