Hepatitis C Virus Subtypes Novel 6g-Related Subtype and 6w Could Be Indigenous in Southern Taiwan with Characteristic Geographic Distribution
- PMID: 34372521
- PMCID: PMC8310057
- DOI: 10.3390/v13071316
Hepatitis C Virus Subtypes Novel 6g-Related Subtype and 6w Could Be Indigenous in Southern Taiwan with Characteristic Geographic Distribution
Abstract
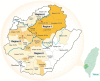
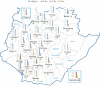
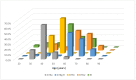
Hepatitis C virus (HCV) genotype (GT) 6 is the most genetically diverse GT and mainly distributed in Southeast Asia and south China but not Taiwan. Earlier studies showed the major HCV GTs in Taiwan were GT 1b and 2 with very rare GT 6 except in injection drug users (IDUs), and subtype 6a is the main GT 6 subtype among IDUs. Recently, we reported a much higher prevalence (18.3%) of GT 6 in Tainan City, southern Taiwan. This study was designed to clarify the subtypes of GT 6 in this endemic area. A total of 3022 (1343 men and 1679 women) HCV viremic patients were enrolled. Subtypes of GT 6 were determined by sequencing of core/E1 and nonstructural protein 5B in 322 of 518 GT 6 patients. The overall GT 6 prevalence rate was 17.1% (518/3022), with higher prevalence districts (>25%) located in northern Tainan. A novel 6g-related subtype is the most prevalent subtype (81.0%), followed by 6w (10.8%), 6a (7.5%), and 6n (0.7%). The high GT 6 prevalence in Tainan was mainly due to a novel 6g-related subtype and 6w. These two subtypes could be indigenous in Tainan with characteristic geographic distribution.
Keywords: genotype; hepatitis C virus; injection drug user; phylogenetic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genetic history of hepatitis C virus genotype 6 in Taiwan.J Formos Med Assoc. 2024 Sep;123(9):926-933. doi: 10.1016/j.jfma.2023.10.013. Epub 2023 Nov 22. J Formos Med Assoc. 2024. PMID: 37996321 Review.
-
High prevalence of genotype 6 hepatitis C virus infection in Southern Taiwan using Abbott genotype assays.J Formos Med Assoc. 2020 Jan;119(1 Pt 3):413-419. doi: 10.1016/j.jfma.2019.07.021. Epub 2019 Aug 13. J Formos Med Assoc. 2020. PMID: 31420113
-
Geographic variation of genotype 6 hepatitis C virus infection in an endemic area of southern Taiwan.J Formos Med Assoc. 2020 Dec;119(12):1876-1880. doi: 10.1016/j.jfma.2020.06.001. Epub 2020 Jun 30. J Formos Med Assoc. 2020. PMID: 32620462
-
Subtype distribution of hepatitis C virus in Jiangsu, China.J Med Virol. 2016 Mar;88(3):498-505. doi: 10.1002/jmv.24356. Epub 2015 Aug 31. J Med Virol. 2016. PMID: 26288243
-
Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution.Virol J. 2017 Feb 23;14(1):41. doi: 10.1186/s12985-017-0710-z. Virol J. 2017. PMID: 28231805 Free PMC article. Review.
Cited by
-
Special Issue "Structural Variations and Molecular Genetics of Hepatitis Virus and Related Viruses".Viruses. 2021 Jul 27;13(8):1456. doi: 10.3390/v13081456. Viruses. 2021. PMID: 34452322 Free PMC article.
References
-
- Childs K., Davis C., Cannon M., Montague S., Filipe A., Tong L., Simmonds P., Smith D., Thomson E.C., Dusheiko G., et al. Suboptimal SVR rates in African patients with atypical genotype 1 subtypes: Implications for global elimination of hepatitis C. J. Hepatol. 2019;71:1099–1105. doi: 10.1016/j.jhep.2019.07.025. - DOI - PMC - PubMed
-
- Nguyen D.T., Tran T.T.T., Nghiem N.M., Le P.T., Vo Q.M., Day J., Rahman M., Le H.M. Effectiveness of sofosbuvir based direct-acting antiviral regimens for chronic hepatitis C virus genotype 6 patients: Real-world experience in Vietnam. PLoS ONE. 2020;15:e0233446. doi: 10.1371/journal.pone.0233446. - DOI - PMC - PubMed
-
- Smith D.B., Davidson F., Simmonds P. Hepatitis C virus variants and the role of genotyping. J. Hepatol. 1995;23(Suppl. 2):26–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

