Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex
- PMID: 34372537
- PMCID: PMC8310193
- DOI: 10.3390/v13071331
Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex
Abstract
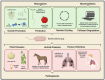
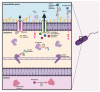
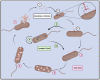
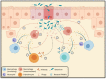
The increasing prevalence and worldwide distribution of multidrug-resistant bacterial pathogens is an imminent danger to public health and threatens virtually all aspects of modern medicine. Particularly concerning, yet insufficiently addressed, are the members of the Burkholderia cepacia complex (Bcc), a group of at least twenty opportunistic, hospital-transmitted, and notoriously drug-resistant species, which infect and cause morbidity in patients who are immunocompromised and those afflicted with chronic illnesses, including cystic fibrosis (CF) and chronic granulomatous disease (CGD). One potential solution to the antimicrobial resistance crisis is phage therapy-the use of phages for the treatment of bacterial infections. Although phage therapy has a long and somewhat checkered history, an impressive volume of modern research has been amassed in the past decades to show that when applied through specific, scientifically supported treatment strategies, phage therapy is highly efficacious and is a promising avenue against drug-resistant and difficult-to-treat pathogens, such as the Bcc. In this review, we discuss the clinical significance of the Bcc, the advantages of phage therapy, and the theoretical and clinical advancements made in phage therapy in general over the past decades, and apply these concepts specifically to the nascent, but growing and rapidly developing, field of Bcc phage therapy.
Keywords: Bcc phage therapy; Burkholderia cepacia complex (Bcc); antibiotic resistance; bacteria; bacteriophages; pathogenesis; phage therapy; phage therapy treatment strategies; phages.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish it.
Figures

References
-
- Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States, 2019. Department of Health and Human Services, CDC; Atlanta, GA, USA: 2019. AR Threats Report.
-
- O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance. Welcome Trust; London, UK: 2014.
-
- Council of Canadian Academies When Antibiotics Fail. [(accessed on 20 June 2021)];2019 Available online: https://www.cca-reports.ca/reports/the-potential-socio-economic-impacts-....
-
- Boucher H.W., Talbot G.H., Benjamin D.K., Bradley J., Guidos R.J., Jones R.N., Murray B.E., Bonomo R.A., Gilbert D. 10 × ′20 progress—Development of new drugs active against gram-negative bacilli: An update from the infectious diseases society of America. Clin. Infect. Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. - DOI - PMC - PubMed
-
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

