Understanding Antibody Responses in Early Life: Baby Steps towards Developing an Effective Influenza Vaccine
- PMID: 34372597
- PMCID: PMC8310046
- DOI: 10.3390/v13071392
Understanding Antibody Responses in Early Life: Baby Steps towards Developing an Effective Influenza Vaccine
Abstract
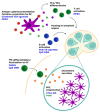
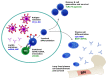
The immune system of young infants is both quantitatively and qualitatively distinct from that of adults, with diminished responsiveness leaving these individuals vulnerable to infection. Because of this, young infants suffer increased morbidity and mortality from respiratory pathogens such as influenza viruses. The impaired generation of robust and persistent antibody responses in these individuals makes overcoming this increased vulnerability through vaccination challenging. Because of this, an effective vaccine against influenza viruses in infants under 6 months is not available. Furthermore, vaccination against influenza viruses is challenging even in adults due to the high antigenic variability across viral strains, allowing immune evasion even after induction of robust immune responses. This has led to substantial interest in understanding how specific antibody responses are formed to variable and conserved components of influenza viruses, as immune responses tend to strongly favor recognition of variable epitopes. Elicitation of broadly protective antibody in young infants, therefore, requires that both the unique characteristics of young infant immunity as well as the antibody immunodominance present among epitopes be effectively addressed. Here, we review our current understanding of the antibody response in newborns and young infants and discuss recent developments in vaccination strategies that can modulate both magnitude and epitope specificity of IAV-specific antibody.
Keywords: B cell; Tfh; antibody; influenza virus; newborn; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vono M., Eberhardt C.S., Auderset F., Mastelic-Gavillet B., Lemeille S., Christensen D., Andersen P., Lambert P.H., Siegrist C.A. Maternal antibodies inhibit neonatal and infant responses to vaccination by shaping the early-life B cell repertoire within germinal centers. Cell. Rep. 2019;28:1773–1784.e1775. doi: 10.1016/j.celrep.2019.07.047. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

