Cross-Reaction or Co-Infection? Serological Discrimination of Antibodies Directed against Dugbe and Crimean-Congo Hemorrhagic Fever Orthonairovirus in Nigerian Cattle
- PMID: 34372604
- PMCID: PMC8310240
- DOI: 10.3390/v13071398
Cross-Reaction or Co-Infection? Serological Discrimination of Antibodies Directed against Dugbe and Crimean-Congo Hemorrhagic Fever Orthonairovirus in Nigerian Cattle
Abstract
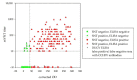
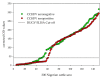
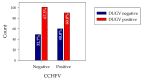
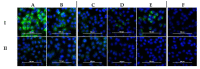
Dugbe orthonairovirus (DUGV) and Crimean-Congo hemorrhagic fever orthonairovirus (CCHFV) are tick-borne arboviruses within the order Bunyavirales. Both viruses are endemic in several African countries and can induce mild (DUGV, BSL 3) or fatal (CCHFV, BSL 4) disease in humans. Ruminants play a major role in their natural transmission cycle. Therefore, they are considered as suitable indicator animals for serological monitoring studies to assess the risk for human infections. Although both viruses do not actually belong to the same serogroup, cross-reactivities have already been reported earlier-hence, the correct serological discrimination of DUGV and CCHFV antibodies is crucial. In this study, 300 Nigerian cattle sera (150 CCHFV seropositive and seronegative samples, respectively) were screened for DUGV antibodies via N protein-based ELISA, indirect immunofluorescence (iIFA) and neutralization assays. Whereas no correlation between the CCHFV antibody status and DUGV seroprevalence data could be demonstrated with a newly established DUGV ELISA, significant cross-reactivities were observed in an immunofluorescence assay. Moreover, DUGV seropositive samples did also cross-react in a species-adapted commercial CCHFV iIFA. Therefore, ELISAs seem to be able to reliably differentiate between DUGV and CCHFV antibodies and should preferentially be used for monitoring studies. Positive iIFA results should always be confirmed by ELISAs.
Keywords: CCHFV; Crimean-Congo hemorrhagic fever orthonairovirus; DUGV; Dugbe orthonairovirus; Nigeria; cattle; cross-reactivity; sensitivity; serology; specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Serological evidence of tick-borne Crimean-Congo haemorrhagic fever and Dugbe orthonairovirus infections in cattle in Kwara State in northern Nigeria indicate independent endemics.PLoS Negl Trop Dis. 2024 Oct 21;18(10):e0012539. doi: 10.1371/journal.pntd.0012539. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39432870 Free PMC article.
-
Experimental Challenge of Sheep and Cattle with Dugbe Orthonairovirus, a Neglected African Arbovirus Distantly Related to CCHFV.Viruses. 2021 Feb 26;13(3):372. doi: 10.3390/v13030372. Viruses. 2021. PMID: 33652845 Free PMC article.
-
Zoonotic arbovirus infections in cattle in Mozambique with special reference to Crimean-Congo hemorrhagic fever virus (CCHFV) and rift valley fever virus (RVFV).Virol J. 2025 Jun 6;22(1):185. doi: 10.1186/s12985-025-02804-9. Virol J. 2025. PMID: 40481487 Free PMC article.
-
Worldwide epidemiology of Crimean-Congo Hemorrhagic Fever Virus in humans, ticks and other animal species, a systematic review and meta-analysis.PLoS Negl Trop Dis. 2021 Apr 22;15(4):e0009299. doi: 10.1371/journal.pntd.0009299. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33886556 Free PMC article.
-
Crimean-Congo Hemorrhagic Fever Virus: Current Advances and Future Prospects of Antiviral Strategies.Viruses. 2021 Jun 22;13(7):1195. doi: 10.3390/v13071195. Viruses. 2021. PMID: 34206476 Free PMC article. Review.
Cited by
-
First evidence of Crimean-Congo haemorrhagic fever virus circulation in Bosnia and Herzegovina.Vet Med Sci. 2022 May;8(3):1271-1275. doi: 10.1002/vms3.781. Epub 2022 Mar 8. Vet Med Sci. 2022. PMID: 35263508 Free PMC article.
-
Seromolecular survey and risk factor analysis of Crimean-Congo haemorrhagic fever orthonairovirus in occupationally exposed herdsmen and unexposed febrile patients in Kwara State, Nigeria.PLoS One. 2024 May 9;19(5):e0303099. doi: 10.1371/journal.pone.0303099. eCollection 2024. PLoS One. 2024. PMID: 38723009 Free PMC article.
-
Serological cross-reactivity between Crimean-Congo haemorrhagic fever virus and Nairobi sheep disease virus glycoprotein C.Front Immunol. 2025 Jan 20;15:1423474. doi: 10.3389/fimmu.2024.1423474. eCollection 2024. Front Immunol. 2025. PMID: 39902044 Free PMC article.
-
Transmission Cycle of Tick-Borne Infections and Co-Infections, Animal Models and Diseases.Pathogens. 2022 Nov 8;11(11):1309. doi: 10.3390/pathogens11111309. Pathogens. 2022. PMID: 36365060 Free PMC article. Review.
-
The Seroprevalence of Crimean-Congo Hemorrhagic Fever in Wild and Domestic Animals: An Epidemiological Update for Domestic Animals and First Seroevidence in Wild Animals from Turkiye.Vet Sci. 2022 Aug 29;9(9):462. doi: 10.3390/vetsci9090462. Vet Sci. 2022. PMID: 36136678 Free PMC article.
References
-
- Causey O.R., Kemp G.E., Casals J., Williams R.W., Madbouly M.H. Dugbe Virus, A New Arbovirus from Nigeria. Niger. J. Sci. 1971;5:41–43.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

