The Role of Coinfections in the EBV-Host Broken Equilibrium
- PMID: 34372605
- PMCID: PMC8310153
- DOI: 10.3390/v13071399
The Role of Coinfections in the EBV-Host Broken Equilibrium
Abstract
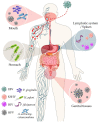
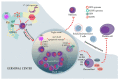
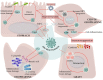
The Epstein-Barr virus (EBV) is a well-adapted human virus, and its infection is exclusive to our species, generally beginning in the childhood and then persisting throughout the life of most of the affected adults. Although this infection generally remains asymptomatic, EBV can trigger life-threatening conditions under unclear circumstances. The EBV lifecycle is characterized by interactions with other viruses or bacteria, which increases the probability of awakening its pathobiont capacity. For instance, EBV infects B cells with the potential to alter the germinal center reaction (GCR)-an adaptive immune structure wherein mutagenic-driven processes take place. HIV- and Plasmodium falciparum-induced B cell hyperactivation also feeds the GCR. These agents, along with the B cell tropic KSHV, converge in the ontogeny of germinal center (GC) or post-GC lymphomas. EBV oral transmission facilitates interactions with local bacteria and HPV, thereby increasing the risk of periodontal diseases and head and neck carcinomas. It is less clear as to how EBV is localized in the stomach, but together with Helicobacter pylori, they are known to be responsible for gastric cancer. Perhaps this mechanism is reminiscent of the local inflammation that attracts different herpesviruses and enhances graft damage and chances of rejection in transplanted patients. In this review, we discussed the existing evidence suggestive of EBV possessing the potential to synergize or cooperate with these agents to trigger or worsen the disease.
Keywords: EBV; H. pylori; HIV; HPV; P. falciparum; beta herpesvirus; coinfection; immunosuppression; lymphomagenesis; periodontal bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Helicobacter pylori and Epstein-Barr Virus Coinfection Stimulates Aggressiveness in Gastric Cancer through the Regulation of Gankyrin.mSphere. 2021 Oct 27;6(5):e0075121. doi: 10.1128/mSphere.00751-21. Epub 2021 Sep 29. mSphere. 2021. PMID: 34585958 Free PMC article.
-
Status of kinases in Epstein-Barr virus and Helicobacter pylori Coinfection in gastric Cancer cells.BMC Cancer. 2020 Sep 29;20(1):925. doi: 10.1186/s12885-020-07377-0. BMC Cancer. 2020. PMID: 32993565 Free PMC article.
-
Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas.World J Gastroenterol. 2018 Nov 21;24(43):4928-4938. doi: 10.3748/wjg.v24.i43.4928. World J Gastroenterol. 2018. PMID: 30487702 Free PMC article.
-
Xenophagy in Helicobacter pylori- and Epstein-Barr virus-induced gastric cancer.J Pathol. 2014 Jun;233(2):103-12. doi: 10.1002/path.4351. J Pathol. 2014. PMID: 24633785 Review.
-
Gastrointestinal inflammation and cancer: viral and bacterial interplay.Gut Microbes. 2025 Dec;17(1):2519703. doi: 10.1080/19490976.2025.2519703. Epub 2025 Jun 26. Gut Microbes. 2025. PMID: 40568785 Free PMC article. Review.
Cited by
-
Molecular Detection of HPV, EBV, HSV-1, HCMV, and H. pylori Pathogens: An Evaluation among Polish Children with Molar Incisor Hypomineralization (MIH).Pathogens. 2024 Apr 22;13(4):345. doi: 10.3390/pathogens13040345. Pathogens. 2024. PMID: 38668300 Free PMC article.
-
Epstein-Barr Virus in the RIVERA Case-Control Study of Acute Febrile Illness: Acute Mononucleosis Nearly Absent as an Etiology in the Peruvian Amazon.Am J Trop Med Hyg. 2024 Nov 12;112(1):200-207. doi: 10.4269/ajtmh.24-0051. Print 2025 Jan 8. Am J Trop Med Hyg. 2024. PMID: 39531721 Free PMC article.
-
Co-infection and co-localization of Kaposi sarcoma-associated herpesvirus and Epstein-Barr virus in HIV-associated Kaposi sarcoma: a case report.Front Cell Infect Microbiol. 2023 Oct 20;13:1270935. doi: 10.3389/fcimb.2023.1270935. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37928187 Free PMC article.
-
The Prevalence of EBV and KSHV in Odontogenic Lesions.Int Dent J. 2023 Feb;73(1):42-47. doi: 10.1016/j.identj.2022.06.028. Epub 2022 Jul 28. Int Dent J. 2023. PMID: 35907672 Free PMC article.
-
Epstein-Barr Virus Infection Is Associated with Elevated Hepcidin Levels.Int J Mol Sci. 2023 Jan 13;24(2):1630. doi: 10.3390/ijms24021630. Int J Mol Sci. 2023. PMID: 36675141 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

