Evolution towards Virulence in a Burkholderia Two-Component System
- PMID: 34372701
- PMCID: PMC8406202
- DOI: 10.1128/mBio.01823-21
Evolution towards Virulence in a Burkholderia Two-Component System
Abstract
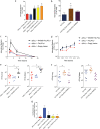
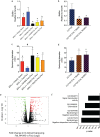
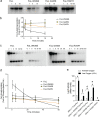
Bacteria in the Burkholderia cepacia complex (BCC) are significant pathogens for people with cystic fibrosis (CF) and are often extensively antibiotic resistant. Here, we assess the impacts of clinically observed mutations in fixL, which encodes the sensor histidine kinase FixL. FixL along with FixJ compose a two-component system that regulates multiple phenotypes. Mutations in fixL across two species, B. dolosa and B. multivorans, have shown evidence of positive selection during chronic lung infection in CF. Herein, we find that BCC carrying the conserved, ancestral fixL sequence have lower survival in macrophages and in murine pneumonia models than mutants carrying evolved fixL sequences associated with clinical decline in CF patients. In vitro phosphotransfer experiments found that one evolved FixL protein, W439S, has a reduced ability to autophosphorylate and phosphorylate FixJ, while LacZ reporter experiments demonstrate that B. dolosa carrying evolved fixL alleles has reduced fix pathway activity. Interestingly, B. dolosa carrying evolved fixL alleles was less fit in a soil assay than those strains carrying the ancestral allele, demonstrating that increased survival of these variants in macrophages and the murine lung comes at a potential expense in their environmental reservoir. Thus, modulation of the two-component system encoded by fixLJ by point mutations is one mechanism that allows BCC to adapt to the host infection environment. IMPORTANCE Infections caused by members of the Burkholderia cepacia complex (BCC) are a serious concern for patients with cystic fibrosis (CF) as these bacteria are often resistant to many antibiotics. During long-term infection of CF patients with BCC, mutations in genes encoding the FixLJ system often become prevalent, suggesting that these changes may benefit the bacteria during infection. The system encoded by fixLJ is involved in sensing oxygen and regulating many genes in response and is required for full virulence of the bacteria in a murine pneumonia model. Evolved fixL mutations seen later in infection improve bacterial persistence within macrophages and enhance infection within mice. However, these adaptations are short sighted because they reduce bacterial fitness within their natural habitat, soil.
Keywords: Burkholderia; evolution; two-component regulatory systems; virulence regulation.
Figures

References
-
- Zlosnik JEA, Zhou G, Brant R, Henry DA, Hird TJ, Mahenthiralingam E, Chilvers MA, Wilcox P, Speert DP. 2015. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years’ experience. Ann Am Thorac Soc 12:70–78. doi:10.1513/AnnalsATS.201408-395OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

