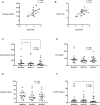
Iron parameters in patients with partial lipodystrophy and impact of exogenous leptin therapy
- PMID: 34373262
- PMCID: PMC8354259
- DOI: 10.1136/bmjdrc-2021-002385
Iron parameters in patients with partial lipodystrophy and impact of exogenous leptin therapy
Keywords: leptin.
Conflict of interest statement
Competing interests: EAO reports the following conflicts: grant support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Ionis Pharmaceuticals, Akcea Therapeutics, Gemphire Therapeutics, GI Dynamics (current), AstraZeneca (past 2 years); consultant or advisor: AstraZeneca, Thera Therapeutics, and BMS (past), Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Regeneron Pharmaceuticals (current); drug support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Akcea Therapeutics, Rhythm Pharmaceuticals (all current); other support: Aegerion Pharmaceuticals (now Amryt Pharmaceuticals), Regeneron Pharmaceuticals (current). BA has attended Scientific Advisory Board Meetings organized by Aegerion Pharmaceuticals (now Amryt Pharmaceuticals) and Regeneron Pharmaceuticals and has received honoraria as a speaker from AstraZeneca, Lilly, MSD, Novartis, Novo Nordisk, Boehringer-Ingelheim, Servier, and Sanofi-Aventis. Other authors report no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
