The Pediatric Precision Oncology INFORM Registry: Clinical Outcome and Benefit for Patients with Very High-Evidence Targets
- PMID: 34373263
- PMCID: PMC9414287
- DOI: 10.1158/2159-8290.CD-21-0094
The Pediatric Precision Oncology INFORM Registry: Clinical Outcome and Benefit for Patients with Very High-Evidence Targets
Abstract
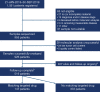
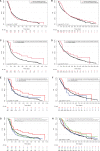
INFORM is a prospective, multinational registry gathering clinical and molecular data of relapsed, progressive, or high-risk pediatric patients with cancer. This report describes long-term follow-up of 519 patients in whom molecular alterations were evaluated according to a predefined seven-scale target prioritization algorithm. Mean turnaround time from sample receipt to report was 25.4 days. The highest target priority level was observed in 42 patients (8.1%). Of these, 20 patients received matched targeted treatment with a median progression-free survival of 204 days [95% confidence interval (CI), 99-not applicable], compared with 117 days (95% CI, 106-143; P = 0.011) in all other patients. The respective molecular targets were shown to be predictive for matched treatment response and not prognostic surrogates for improved outcome. Hereditary cancer predisposition syndromes were identified in 7.5% of patients, half of which were newly identified through the study. Integrated molecular analyses resulted in a change or refinement of diagnoses in 8.2% of cases. SIGNIFICANCE: The pediatric precision oncology INFORM registry prospectively tested a target prioritization algorithm in a real-world, multinational setting and identified subgroups of patients benefiting from matched targeted treatment with improved progression-free survival, refinement of diagnosis, and identification of hereditary cancer predisposition syndromes.See related commentary by Eggermont et al., p. 2677.This article is highlighted in the In This Issue feature, p. 2659.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Precision Cancer Medicine: Large Studies Indicate Steady Progress.Cancer Discov. 2021 Nov;11(11):2677-2678. doi: 10.1158/2159-8290.CD-21-1069. Cancer Discov. 2021. PMID: 34725088
References
-
- Antony R, Wong KE, Patel M, Olch AJ, McComb G, Krieger Met al. . A retrospective analysis of recurrent intracranial ependymoma. Pediatr Blood Cancer 2014;61:1195–201. - PubMed
-
- Athale UH, Duckworth J, Odame I, Barr R. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol 2009;31:651–63. - PubMed
-
- Bautista F, Di Giannatale A, Dias-Gastellier N, Fahd M, Valteau-Couanet D, Couanet Det al. . Patients in pediatric phase I and early phase II clinical oncology trials at Gustave Roussy: a 13-year center experience. J Pediatr Hematol Oncol 2015;37:e102–10. - PubMed
-
- Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GUet al. . Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005;23:559–68. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

