Rationale and design of the IRON-AF study: a double-blind, randomised, placebo-controlled study to assess the effect of intravenous ferric carboxymaltose in patients with atrial fibrillation and iron deficiency
- PMID: 34373301
- PMCID: PMC8354291
- DOI: 10.1136/bmjopen-2020-047642
Rationale and design of the IRON-AF study: a double-blind, randomised, placebo-controlled study to assess the effect of intravenous ferric carboxymaltose in patients with atrial fibrillation and iron deficiency
Abstract
Introduction: Atrial fibrillation (AF) is associated with significantly impaired quality-of-life. Iron deficiency (ID) is prevalent in patients with AF. Correction of ID in other patient populations with intravenous iron supplementation has been shown to be a safe, convenient and effective way of improving exercise tolerance, fatigue and quality-of-life. The IRON-AF (Effect of Iron Repletion in Atrial Fibrillation) study is designed to assess the effect of iron repletion with intravenous ferric carboxymaltose in patients with AF and ID.
Methods and analysis: The IRON-AF study is a double-blind, randomised controlled trial that will recruit at least 84 patients with AF and ID. Patients will be randomised to receive infusions of either ferric carboxymaltose or placebo, given in repletion and then maintenance doses. The study will have follow-up visits at weeks 4, 8 and 12. The primary endpoint is change in peak oxygen uptake from baseline to week 12, as measured by cardiopulmonary exercise testing (CPET) on a cycle ergometer. Secondary endpoints include changes in quality-of-life and AF disease burden scores, blood parameters, other CPET parameters, transthoracic echocardiogram parameters, 6-minute walk test distance, 7-day Holter/Event monitor burden of AF, health resource utilisation and mortality.
Ethics and dissemination: The study protocol has been approved by the Central Adelaide Local Health Network Human Research Ethics Committee, Australia. The results of this study will be disseminated through publications in peer-reviewed journals and conference presentations.
Trial registration number: Australian New Zealand Clinical Trials Registry (ACTRN12620000285954).
Keywords: cardiology; clinical trials; pacing & electrophysiology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DL reports the University of Adelaide has received on his behalf lecture and/or consulting fees from Abbott Medical, Bayer, Biotronik, Boehringer Ingelheim, Medtronic, Microport and Pfizer/BMS. PS reports having served on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx and PaceMate. PS reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott Medical and Boston Scientific. PS reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott Medical, Boston Scientific and Microport. CXW reports that the University of Adelaide has received on his behalf lecture, travel and/or research funding from Abbott Medical, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, St Jude Medical and Vifor Pharma.
Figures

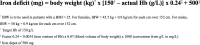
Similar articles
-
Effect of Intravenous Ferric Carboxymaltose on Exercise Capacity After Kidney Transplantation (EFFECT-KTx): rationale and study protocol for a double-blind, randomised, placebo-controlled trial.BMJ Open. 2023 Mar 22;13(3):e065423. doi: 10.1136/bmjopen-2022-065423. BMJ Open. 2023. PMID: 36948568 Free PMC article.
-
Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure.Eur J Heart Fail. 2019 Dec;21(12):1651-1658. doi: 10.1002/ejhf.1710. Epub 2019 Dec 28. Eur J Heart Fail. 2019. PMID: 31883356 Clinical Trial.
-
Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial.Lancet Haematol. 2016 Sep;3(9):e415-25. doi: 10.1016/S2352-3026(16)30078-3. Epub 2016 Aug 4. Lancet Haematol. 2016. PMID: 27570088 Clinical Trial.
-
Ferric carboxymaltose: a review of its use in iron deficiency.Drugs. 2015 Jan;75(1):101-27. doi: 10.1007/s40265-014-0332-3. Drugs. 2015. PMID: 25428711 Review.
-
Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis.Eur J Heart Fail. 2018 Jan;20(1):125-133. doi: 10.1002/ejhf.823. Epub 2017 Apr 24. Eur J Heart Fail. 2018. PMID: 28436136 Review.
Cited by
-
Iron deficiency and supplementation in heart failure.Nat Rev Cardiol. 2024 Jul;21(7):463-486. doi: 10.1038/s41569-024-00988-1. Epub 2024 Feb 7. Nat Rev Cardiol. 2024. PMID: 38326440 Review.
-
[Iron deficiency in cardiovascular disease].Inn Med (Heidelb). 2024 Dec;65(12):1273-1282. doi: 10.1007/s00108-024-01783-3. Epub 2024 Sep 30. Inn Med (Heidelb). 2024. PMID: 39349882 Review. German.
-
Iron deficiency and cardiovascular disease.Eur Heart J. 2023 Jan 1;44(1):14-27. doi: 10.1093/eurheartj/ehac569. Eur Heart J. 2023. PMID: 36282723 Free PMC article.
-
Improvement in left atrial strain following ferric carboxymaltose in heart failure: an analysis of the Myocardial-IRON trial.ESC Heart Fail. 2024 Apr;11(2):1258-1262. doi: 10.1002/ehf2.14630. Epub 2023 Dec 20. ESC Heart Fail. 2024. PMID: 38115745 Free PMC article. Clinical Trial.
-
The Mutual Relationship among Cardiovascular Diseases and COVID-19: Focus on Micronutrients Imbalance.Nutrients. 2022 Aug 21;14(16):3439. doi: 10.3390/nu14163439. Nutrients. 2022. PMID: 36014944 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
