High-throughput dissection of the thermodynamic and conformational properties of a ubiquitous class of RNA tertiary contact motifs
- PMID: 34373334
- PMCID: PMC8379967
- DOI: 10.1073/pnas.2109085118
High-throughput dissection of the thermodynamic and conformational properties of a ubiquitous class of RNA tertiary contact motifs
Abstract
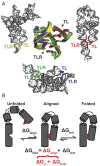
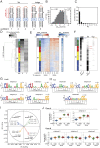
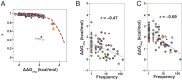
Despite RNA's diverse secondary and tertiary structures and its complex conformational changes, nature utilizes a limited set of structural "motifs"-helices, junctions, and tertiary contact modules-to build diverse functional RNAs. Thus, in-depth descriptions of a relatively small universe of RNA motifs may lead to predictive models of RNA tertiary conformational landscapes. Motifs may have different properties depending on sequence and secondary structure, giving rise to subclasses that expand the universe of RNA building blocks. Yet we know very little about motif subclasses, given the challenges in mapping conformational properties in high throughput. Previously, we used "RNA on a massively parallel array" (RNA-MaP), a quantitative, high-throughput technique, to study thousands of helices and two-way junctions. Here, we adapt RNA-MaP to study the thermodynamic and conformational properties of tetraloop/tetraloop receptor (TL/TLR) tertiary contact motifs, analyzing 1,493 TLR sequences from different classes. Clustering analyses revealed variability in TL specificity, stability, and conformational behavior. Nevertheless, natural GAAA/11ntR TL/TLRs, while varying in tertiary stability by ∼2.5 kcal/mol, exhibited conserved TL specificity and conformational properties. Thus, RNAs may tune stability without altering the overall structure of these TL/TLRs. Furthermore, their stability correlated with natural frequency, suggesting thermodynamics as the dominant selection pressure. In contrast, other TL/TLRs displayed heterogenous conformational behavior and appear to not be under strong thermodynamic selection. Our results build toward a generalizable model of RNA-folding thermodynamics based on the properties of isolated motifs, and our characterized TL/TLR library can be used to engineer RNAs with predictable thermodynamic and conformational behavior.
Keywords: RNA folding; RNA nanotechnology; RNA structure; high-throughput biochemistry; tertiary motifs.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

