Prognostic Accuracy of NEDA-3 in Long-term Outcomes of Multiple Sclerosis
- PMID: 34373345
- PMCID: PMC8353667
- DOI: 10.1212/NXI.0000000000001059
Prognostic Accuracy of NEDA-3 in Long-term Outcomes of Multiple Sclerosis
Abstract
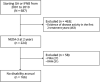
Background and objectives: To estimate the proportions of patients with relapsing-remitting multiple sclerosis who despite achieving the no evidence of disease activity-3 (NEDA-3) status in the first 2 treatment years experienced relapse-associated worsening (RAW) or progression independent from relapse activity (PIRA) in the following years.
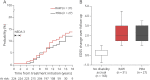
Methods: We selected patients with NEDA-3-defined as no relapse, no disability worsening, and no MRI activity-in the first 2 years of either glatiramer acetate or interferon beta as initial treatment. We estimated the long-term probability of subsequent RAW and PIRA (considered as 2 contrasting outcomes) by cumulative incidence functions. Competing risk regressions were used to identify the baseline (i.e., at treatment start) predictors of RAW and PIRA.
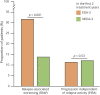
Results: Of 687 patients, 224 (32.6%) had NEDA-3 in the first 2 treatment years. After a median follow-up time of 12 years from treatment start, 58 patients (26%) experienced disability accrual: 31 (14%) had RAW and 27 (12%) had PIRA. RAW was predicted by the presence of >9 T2 lesions (subdistribution hazard ratio [SHR] = 3.92, p = 0.012) and contrast-enhancing lesions (SHR = 2.38, p = 0.047) on baseline MRI scan and either temporary or permanent discontinuation of the initial treatment (SHR = 1.11, p = 0.015). PIRA was predicted by advancing age (SHR = 1.05, p = 0.036 for each year increase) and presence of ≥1 spinal cord lesion on baseline MRI scan (SHR = 4.08, p = 0.016).
Discussion: The adoption of NEDA-3 criteria led to prognostic misclassification in 1 of 4 patients. Different risk factors were associated with RAW and PIRA, suggesting alternative mechanisms for disability accrual.
Classification of evidence: This study provides Class II evidence that in patients with RRMS who attained NEDA-3 status, subsequent RAW was associated with baseline MRI activity and discontinuation of treatment and PIRA was associated with age and the presence of baseline spinal cord lesions.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of No evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72(2):152-158. - PubMed
-
- Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
