Connecting primitive phase separation to biotechnology, synthetic biology, and engineering
- PMID: 34373367
- PMCID: PMC8342986
- DOI: 10.1007/s12038-021-00204-z
Connecting primitive phase separation to biotechnology, synthetic biology, and engineering
Abstract
One aspect of the study of the origins of life focuses on how primitive chemistries assembled into the first cells on Earth and how these primitive cells evolved into modern cells. Membraneless droplets generated from liquid-liquid phase separation (LLPS) are one potential primitive cell-like compartment; current research in origins of life includes study of the structure, function, and evolution of such systems. However, the goal of primitive LLPS research is not simply curiosity or striving to understand one of life's biggest unanswered questions, but also the possibility to discover functions or structures useful for application in the modern day. Many applicational fields, including biotechnology, synthetic biology, and engineering, utilize similar phaseseparated structures to accomplish specific functions afforded by LLPS. Here, we briefly review LLPS applied to primitive compartment research and then present some examples of LLPS applied to biomolecule purification, drug delivery, artificial cell construction, waste and pollution management, and flavor encapsulation. Due to a significant focus on similar functions and structures, there appears to be much for origins of life researchers to learn from those working on LLPS in applicational fields, and vice versa, and we hope that such researchers can start meaningful cross-disciplinary collaborations in the future.
Figures


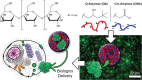
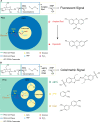
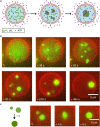
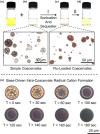
References
-
- Afify A, Gwad EL, AA, and EL Rahman NA, Using Enterobacter aerogenes DSM 30053 for bio-hydrogen production by microbial electrolysis cells from domestic wastewater. J. Agric. Chem. Biotech. 2017;8:167–171.
-
- Alberts B, Johnson AD, Lewis J, Morgan D, Raff M, Roberts K, Walter P. Molecular biology of the cell: sixth international student. New York: W.W. Norton & Company; 2014.
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, and Walter P 2007 Molecular biology of the cell (New York: Garland Science)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
