Coregulation of pathways in lung cancer patients with EGFR mutation: therapeutic opportunities
- PMID: 34373568
- PMCID: PMC8351231
- DOI: 10.1038/s41416-021-01519-2
Coregulation of pathways in lung cancer patients with EGFR mutation: therapeutic opportunities
Abstract
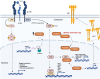
Epidermal growth factor receptor (EGFR) mutations in lung adenocarcinoma are a frequent class of driver mutations. Single EGFR tyrosine kinase inhibitor (TKI) provides substantial clinical benefit, but almost nil radiographic complete responses. Patients invariably progress, although survival can reach several years with post-treatment therapies, including EGFR TKIs, chemotherapy or other procedures. Endeavours have been clinically oriented to manage the acquisition of EGFR TKI-resistant mutations; however, basic principles on cancer evolution have not been considered in clinical trials. For years, evidence has displayed rapidly adaptive mechanisms of resistance to selective monotherapy, posing several dilemmas for the practitioner. Strict adherence to non-small cell lung cancer (NSCLC) guidelines is not always practical for addressing the clinical progression that EGFR-mutant lung adenocarcinoma patients suffer. The purpose of this review is to highlight regulatory mechanisms and signalling pathways that cause therapy-induced resistance to EGFR TKIs. It suggests combinatorial therapies that target EGFR, as well as potential mechanisms underlying EGFR-mutant NSCLC, alerting the reader to clinical opportunities that may lead to a deeper and more durable response. Molecular reprogramming contributes to EGFR TKI resistance, and the compiled information is relevant in understanding the development of new combined targeted strategies in EGFR-mutant NSCLC.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. - PubMed
-
- Sun X, Song Q, He L, Yan L, Liu J, Zhang Q, et al. Receptor tyrosine kinase phosphorylation pattern-based multidrug combination is an effective approach for personalized cancer treatment. Mol Cancer Ther. 2016;15:2508–20. - PubMed
-
- Gusenbauer S, Vlaicu P, Ullrich A. HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene. 2013;32:3846–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

