Inflammasome activation at the crux of severe COVID-19
- PMID: 34373622
- PMCID: PMC8351223
- DOI: 10.1038/s41577-021-00588-x
Inflammasome activation at the crux of severe COVID-19
Abstract
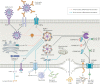
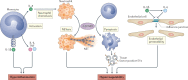
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), results in life-threatening disease in a minority of patients, especially elderly people and those with co-morbidities such as obesity and diabetes. Severe disease is characterized by dysregulated cytokine release, pneumonia and acute lung injury, which can rapidly progress to acute respiratory distress syndrome, disseminated intravascular coagulation, multisystem failure and death. However, a mechanistic understanding of COVID-19 progression remains unclear. Here we review evidence that SARS-CoV-2 directly or indirectly activates inflammasomes, which are large multiprotein assemblies that are broadly responsive to pathogen-associated and stress-associated cellular insults, leading to secretion of the pleiotropic IL-1 family cytokines (IL-1β and IL-18), and pyroptosis, an inflammatory form of cell death. We further discuss potential mechanisms of inflammasome activation and clinical efforts currently under way to suppress inflammation to prevent or ameliorate severe COVID-19.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

