Fructose-coated Ångstrom silver prevents sepsis by killing bacteria and attenuating bacterial toxin-induced injuries
- PMID: 34373734
- PMCID: PMC8344005
- DOI: 10.7150/thno.55334
Fructose-coated Ångstrom silver prevents sepsis by killing bacteria and attenuating bacterial toxin-induced injuries
Abstract
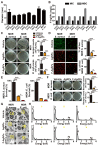
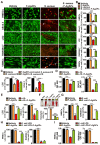
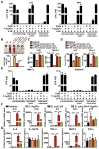
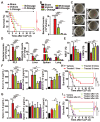
Serious infection caused by multi-drug-resistant bacteria is a major threat to human health. Bacteria can invade the host tissue and produce various toxins to damage or kill host cells, which may induce life-threatening sepsis. Here, we aimed to explore whether fructose-coated Ångstrom-scale silver particles (F-AgÅPs), which were prepared by our self-developed evaporation-condensation system and optimized coating approach, could kill bacteria and sequester bacterial toxins to attenuate fatal bacterial infections. Methods: A series of in vitro assays were conducted to test the anti-bacterial efficacy of F-AgÅPs, and to investigate whether F-AgÅPs could protect against multi-drug resistant Staphylococcus aureus (S. aureus)- and Escherichia coli (E. coli)-induced cell death, and suppress their toxins (S. aureus hemolysin and E. coli lipopolysaccharide)-induced cell injury or inflammation. The mouse models of cecal ligation and puncture (CLP)- or E. coli bloodstream infection-induced lethal sepsis were established to assess whether the intravenous administration of F-AgÅPs could decrease bacterial burden, inhibit inflammation, and improve the survival rates of mice. The levels of silver in urine and feces of mice were examined to evaluate the excretion of F-AgÅPs. Results: F-AgÅPs efficiently killed various bacteria that can cause lethal infections and also competed with host cells to bind with S. aureus α-hemolysin, thus blocking its cytotoxic activity. F-AgÅPs inhibited E. coli lipopolysaccharide-induced endothelial injury and macrophage inflammation, but not by directly binding to lipopolysaccharide. F-AgÅPs potently reduced bacterial burden, reversed dysregulated inflammation, and enhanced survival in mice with CLP- or E. coli bloodstream infection-induced sepsis, either alone or combined with antibiotic therapy. After three times injections within 48 h, 79.18% of F-AgÅPs were excreted via feces at the end of the 14-day observation period. Conclusion: This study suggests the prospect of F-AgÅPs as a promising intravenous agent for treating severe bacterial infections.
Keywords: bacterial infection; inflammation; lipopolysaccharide; Ångstrom-scale silver particles; α-hemolysin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Furst AL, Francis MB. Impedance-based detection of bacteria. Chem Rev. 2019;119:700–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

