Neuronal-driven glioma growth requires Gαi1 and Gαi3
- PMID: 34373757
- PMCID: PMC8343996
- DOI: 10.7150/thno.61452
Neuronal-driven glioma growth requires Gαi1 and Gαi3
Abstract
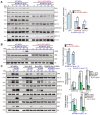
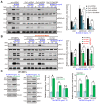
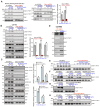
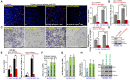
Neuroligin-3 (NLGN3) is necessary and sufficient to promote glioma cell growth. The recruitment of Gαi1/3 to the ligand-activated receptor tyrosine kinases (RTKs) is essential for mediating oncogenic signaling. Methods: Various genetic strategies were utilized to examine the requirement of Gαi1/3 in NLGN3-driven glioma cell growth. Results: NLGN3-induced Akt-mTORC1 and Erk activation was inhibited by decreasing Gαi1/3 expression. In contrast ectopic Gαi1/3 overexpression enhanced NLGN3-induced signaling. In glioma cells, NLGN3-induced cell growth, proliferation and migration were attenuated by Gαi1/3 depletion with shRNA, but facilitated with Gαi1/3 overexpression. Significantly, Gαi1/3 silencing inhibited orthotopic growth of patient-derived glioma xenografts in mouse brain, whereas forced Gαi1/3-overexpression in primary glioma xenografts significantly enhanced growth. The growth of brain-metastatic human lung cancer cells in mouse brain was largely inhibited with Gαi1/3 silencing. It was however expedited with ectopic Gαi1/3 overexpression. In human glioma Gαi3 upregulation was detected, correlating with poor prognosis. Conclusion: Gαi1/3 mediation of NLGN3-induced signaling is essential for neuronal-driven glioma growth.
Keywords: Gαi1/3; NLGN3; Neuron-glioma communication; Signaling.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Reardon DA, Wen PY. Glioma in 2014: unravelling tumour heterogeneity-implications for therapy. Nat Rev Clin Oncol. 2015;12:69–70. - PubMed
-
- Wen PY, Reardon DA. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12:69–70. - PubMed
-
- Kwiatkowska A, Symons M. Signaling Determinants of Glioma Cell Invasion. Adv Exp Med Biol. 2020;1202:129–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

