Pulmonary circulation-mediated heart targeting for the prevention of heart failure by inhalation of intrinsically bioactive nanoparticles
- PMID: 34373758
- PMCID: PMC8343995
- DOI: 10.7150/thno.61875
Pulmonary circulation-mediated heart targeting for the prevention of heart failure by inhalation of intrinsically bioactive nanoparticles
Abstract
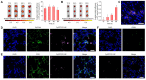
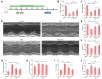
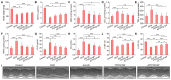
Heart failure is a serious clinical and public health problem. Currently there is an unmet demand for effective therapies for heart failure. Herein we reported noninvasive inhalation delivery of nanotherapies to prevent heart failure. Methods: A reactive oxygen species (ROS)-scavenging material (TPCD) was synthesized, which was processed into antioxidative and anti-inflammatory nanoparticles (i.e., TPCD NP). By decoration with a mitochondrial-targeting moiety, a multilevel targeting nanotherapy TTPCD NP was engineered. Pulmonary accumulation of inhaled TPCD NP and underlying mechanisms were examined in mice. In vivo efficacies of nanotherapies were evaluated in mice with doxorubicin (DOX)-induced cardiomyopathy. Further, an antioxidative, anti-inflammatory, and pro-resolving nanotherapy (i.e., ATTPCD NP) was developed, by packaging a peptide Ac2-26. In vitro and in vivo efficacies of ATTPCD NP were also evaluated. Results: TPCD NP alleviated DOX-induced oxidative stress and cell injury by internalization in cardiomyocytes and scavenging overproduced ROS. Inhaled TPCD NP can accumulate in the heart of mice by transport across the lung epithelial and endothelial barriers. Correspondingly, inhaled TPCD NP effectively inhibited DOX-induced heart failure in mice. TTPCD NP showed considerably enhanced heart targeting capability, cellular uptake efficiency, and mitochondrial localization capacity, thereby potentiating therapeutic effects. Notably, TPCD NP can serve as bioactive and ROS-responsive nanovehicles to achieve combination therapy with Ac2-26, affording further enhanced efficacies. Importantly, inhaled TPCD NP displayed good safety at a dose 5-fold higher than the efficacious dose. Conclusions: Inhalation delivery of nanoparticles is an effective, safe, and noninvasive strategy for targeted treatment of heart diseases. TPCD NP-based nanotherapies are promising drugs for heart failure and other acute/chronic heart diseases associated with oxidative stress.
Keywords: bioactive nanoparticles; cardiac dysfunction; heart failure; inhalation delivery; nanotherapy; targeted therapy.
© The author(s).
Conflict of interest statement
Competing Interests: C.L., Y.M., L.L., H.H., and J.Z. are inventors in a pending patent filed by the National Intellectual Property Administration of the PRC (No. 202110601623.4, 31 May 2021) related to mitochondrial-targeting Ac2-26/TPCD nanotherapies for the prevention of heart failure, but the rights belong to Third Military Medical University (Army Medical University). All other authors declare that they have no competing interests.
Figures







Similar articles
-
Targeted Treatment of Ischemic Stroke by Bioactive Nanoparticle-Derived Reactive Oxygen Species Responsive and Inflammation-Resolving Nanotherapies.ACS Nano. 2021 Oct 26;15(10):16076-16094. doi: 10.1021/acsnano.1c04753. Epub 2021 Oct 4. ACS Nano. 2021. PMID: 34606239
-
Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity.ACS Nano. 2018 Sep 25;12(9):8943-8960. doi: 10.1021/acsnano.8b02037. Epub 2018 Aug 21. ACS Nano. 2018. PMID: 30114351
-
A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases.Biomaterials. 2020 Feb;230:119605. doi: 10.1016/j.biomaterials.2019.119605. Epub 2019 Nov 8. Biomaterials. 2020. PMID: 31740099
-
Nanomedicine for the treatment of diabetes-associated cardiovascular diseases and fibrosis.Adv Drug Deliv Rev. 2021 May;172:234-248. doi: 10.1016/j.addr.2021.01.004. Epub 2021 Jan 5. Adv Drug Deliv Rev. 2021. PMID: 33417981 Review.
-
Aptamers in targeted nanotherapy.Curr Top Med Chem. 2015;15(12):1102-14. doi: 10.2174/1568026615666150413153525. Curr Top Med Chem. 2015. PMID: 25866269 Review.
Cited by
-
The applications of functional materials-based nano-formulations in the prevention, diagnosis and treatment of chronic inflammation-related diseases.Front Pharmacol. 2023 Aug 1;14:1222642. doi: 10.3389/fphar.2023.1222642. eCollection 2023. Front Pharmacol. 2023. PMID: 37593176 Free PMC article. Review.
-
Autophagy and mitophagy as potential therapeutic targets in diabetic heart condition: Harnessing the power of nanotheranostics.Asian J Pharm Sci. 2024 Jun;19(3):100927. doi: 10.1016/j.ajps.2024.100927. Epub 2024 May 19. Asian J Pharm Sci. 2024. PMID: 38948399 Free PMC article. Review.
-
Designer Functional Nanomedicine for Myocardial Repair by Regulating the Inflammatory Microenvironment.Pharmaceutics. 2022 Mar 31;14(4):758. doi: 10.3390/pharmaceutics14040758. Pharmaceutics. 2022. PMID: 35456592 Free PMC article. Review.
-
Nanoparticles in the diagnosis and treatment of vascular aging and related diseases.Signal Transduct Target Ther. 2022 Jul 11;7(1):231. doi: 10.1038/s41392-022-01082-z. Signal Transduct Target Ther. 2022. PMID: 35817770 Free PMC article. Review.
-
Intravascularly Deliverable Biomaterial Platforms for Tissue Repair and Regeneration Post-Myocardial Infarction.Adv Mater. 2024 Oct;36(43):e2300603. doi: 10.1002/adma.202300603. Epub 2023 Oct 29. Adv Mater. 2024. PMID: 36989469 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

