Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds
- PMID: 34373963
- PMCID: PMC8352847
- DOI: 10.1007/s41061-021-00347-5
Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds
Abstract
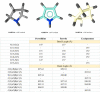
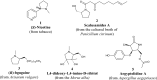
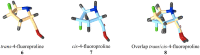
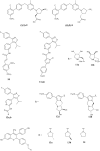
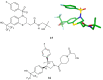
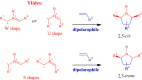
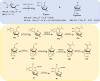
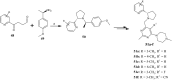
The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases. The great interest in this saturated scaffold is enhanced by (1) the possibility to efficiently explore the pharmacophore space due to sp3-hybridization, (2) the contribution to the stereochemistry of the molecule, (3) and the increased three-dimensional (3D) coverage due to the non-planarity of the ring-a phenomenon called "pseudorotation". In this review, we report bioactive molecules with target selectivity characterized by the pyrrolidine ring and its derivatives, including pyrrolizines, pyrrolidine-2-one, pyrrolidine-2,5-diones and prolinol described in the literature from 2015 to date. After a comparison of the physicochemical parameters of pyrrolidine with the parent aromatic pyrrole and cyclopentane, we investigate the influence of steric factors on biological activity, also describing the structure-activity relationship (SAR) of the studied compounds. To aid the reader's approach to reading the manuscript, we have planned the review on the basis of the synthetic strategies used: (1) ring construction from different cyclic or acyclic precursors, reporting the synthesis and the reaction conditions, or (2) functionalization of preformed pyrrolidine rings, e.g., proline derivatives. Since one of the most significant features of the pyrrolidine ring is the stereogenicity of carbons, we highlight how the different stereoisomers and the spatial orientation of substituents can lead to a different biological profile of drug candidates, due to the different binding mode to enantioselective proteins. We believe that this work can guide medicinal chemists to the best approach in the design of new pyrrolidine compounds with different biological profiles.
Keywords: Anti-inflammatory and analgesic agents; Anticancer and antibacterial agents; Antidiabetics; Central nervous system diseases; Pyrrolidine.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








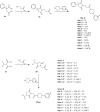





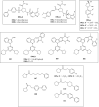


References
-
- Cascioferro S, Maggio B, Raffa D, et al. A new class of phenylhydrazinylidene derivatives as inhibitors of Staphylococcus aureus biofilm formation. Med Chem Res. 2016;25:870–878. doi: 10.1007/s00044-016-1535-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
