Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis
- PMID: 34374109
- PMCID: PMC8692189
- DOI: 10.1111/jvim.16231
Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis
Abstract
Background: Differentiation of low-grade intestinal T-cell lymphoma (LGITL) from lymphoplasmacytic enteritis (LPE) in cats is a diagnostic challenge for pathologists.
Objective: Characterize histologic, immunohistochemical, and molecular features of LGITL and LPE.
Animals: Forty-four client-owned cats, 22 diagnosed with LGITL and 22 with LPE.
Methods: Prospective, cohort study. Clinical suspicion of LGITL or LPE was based on persistent gastrointestinal signs, unresponsive to empirical treatments. All cats underwent a standardized diagnostic evaluation, including biopsy (preferentially full-thickness), and were diagnosed with LGITL or LPE after review of clinical, laboratory, sonographic, histologic, immunohistochemical, and clonality results.
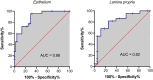
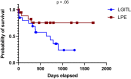
Results: A monomorphic lymphocytic population (22/22, 100%) and in-depth mucosal infiltration (15/22, 68%) were hallmarks of LGITL. Epithelial patterns (nests and plaques) were significantly more frequent in LGITL (11/22, 50%) than in LPE (1/22, 5%) cases (P = .001). A CD3+ lymphocytic apical-to-basal gradient was observed in 9/22 (41%) of LGITL vs 1/22 (5%) of LPE cases (P = .004). Most LPE cases (17/18, 94%) featured marked fibrosis in the superficial part of the lamina propria. The Ki-67 20%- and 30%-thresholds discriminated between LGITL and LPE within both the epithelium (specificity >95%) and lamina propria (specificity >95%), respectively. All LGITL cases were CD3+ pSTAT3- and pSTAT5+. T-cell receptor gamma chain gene rearrangements indicated monoclonality in 86% of LGITL cases. Surprisingly, 70% of LPE cases featured monoclonality (40%) or monoclonality on a polyclonal background (30%).
Conclusions and clinical importance: We identified new histologic, immunohistochemical, and clonality criteria to distinguish LGITL from LPE.
Keywords: CD20; CD3; JAK-STAT; Ki-67; PARR; alimentary lymphoma; clonality; epithelium; fibrosis; full-thickness intestinal biopsies; gradient; histology; immunohistochemistry; inflammatory bowel disease; lamina propria; monoclonal; nest; plaque; polyclonal.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Dr Maria Elena Turba works at Genefast Laboratory (Italy). Otherwise, the authors declare no conflict of interest with respect to the research, authorship, and publication of this article.
Figures


References
-
- Moore PF, Rodriguez‐Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658‐668. - PubMed
-
- Evans SE, Bonczynski JJ, Broussard JD, et al. Comparison of endoscopic and full‐thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447‐1450. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

