A randomized, double blind, single dose, comparative study of the pharmacokinetics, safety and immunogenicity of MB02 (bevacizumab biosimilar) and reference bevacizumab in healthy male volunteers
- PMID: 34374114
- PMCID: PMC9291832
- DOI: 10.1111/bcp.15032
A randomized, double blind, single dose, comparative study of the pharmacokinetics, safety and immunogenicity of MB02 (bevacizumab biosimilar) and reference bevacizumab in healthy male volunteers
Abstract
Aims: The pharmacokinetic (PK) similarity between MB02, a proposed bevacizumab biosimilar, and reference bevacizumab approved from the USA (US-bevacizumab) and European Union (EU-bevacizumab) was evaluated. Safety and immunogenicity were also assessed.
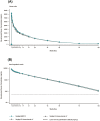
Methods: In this phase 1, randomized, double blind, single dose, parallel group study, 114 healthy male volunteers were randomized 1:1:1 to receive a 3 mg/kg intravenous dose of MB02, US-bevacizumab or EU-bevacizumab, and evaluated for 100 days. PK similarity between MB02 and reference bevacizumab was determined using the standard bioequivalence criteria (0.80-1.25) for the area under the serum concentration-time curve from time 0 extrapolated to infinity (AUC(0-∞) ) and the maximum observed serum concentration (Cmax ).
Results: Baseline demographics were similar across treatment groups. All study drugs exhibited similar PK profile. The 90% confidence interval for the geometric lead square means ratios for the primary parameters AUC(0-∞) and Cmax for MB02, US-bevacizumab and EU-bevacizumab were fully contained within the pre-defined bioequivalence limits for the 3 pairwise comparisons: AUC(0-∞) (MB02:US-bevacizumab 0.998 [0.944 to 1.05]; MB02:EU-bevacizumab 1.07 [1.00 to 1.14]; and US-bevacizumab:EU-bevacizumab 0.934 [0.884 to 0.988]) and Cmax (MB02:US-bevacizumab 0.983 [0.897 to 1.08]; MB02:EU-bevacizumab 1.06 [0.976 to 1.16]; and; US-bevacizumab: EU-bevacizumab 0.926 [0.851 to 1.01]). Treatment emergent adverse events were reported in 87 subjects (76.3%), most being mild and with comparable incidence among treatment groups. Thirty-three subjects (28.9%) reported 56 possibly related treatment emergent adverse events with comparable incidence across treatments, the most frequent being headache (10.5%) and fatigue (3.5%). Anti-drug antibody incidence was low and similar between treatment groups.
Conclusions: This study demonstrates the PK similarity and bioequivalence of MB02 to the reference bevacizumab, whether approved from USA or EU. The safety and immunogenicity profile of MB02 was shown also to be similar to the bevacizumab reference product (NCT04238663).
Keywords: MB02; bevacizumab; biosimilars; pharmacokinetics; safety.
© 2021 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
A. Sinn from Parexel International GmbH declares no potential financial interest in the subject matter or materials discussed in this manuscript. F. García‐Alvarado, V. Gonzalez, C. Huerga and F. Bullo are employees of mAbxience Research S.L.
Figures



Similar articles
-
A randomized, double-blind, single-dose study to assess bioequivalence of MB02 biosimilar after manufacturing iteration and reference bevacizumab.Pharmacol Res Perspect. 2023 Apr;11(2):e01070. doi: 10.1002/prp2.1070. Pharmacol Res Perspect. 2023. PMID: 36914963 Free PMC article. Clinical Trial.
-
Pooled analysis of three pharmacokinetic studies comparing biosimilar MB02 and reference bevacizumab.Pharmacol Res Perspect. 2023 Dec;11(6):e01139. doi: 10.1002/prp2.1139. Pharmacol Res Perspect. 2023. PMID: 37920875 Free PMC article.
-
A randomized, single-dose, pharmacokinetic equivalence study comparing MB02 (proposed biosimilar) and reference bevacizumab in healthy Japanese male volunteers.Cancer Chemother Pharmacol. 2021 Oct;88(4):713-722. doi: 10.1007/s00280-021-04324-z. Epub 2021 Jul 16. Cancer Chemother Pharmacol. 2021. PMID: 34269848 Free PMC article. Clinical Trial.
-
Optimizing biosimilar development: current approaches to demonstrating pharmacokinetic and analytical similarity and a proposal for a single reference approach.Expert Opin Biol Ther. 2025 Apr;25(4):447-454. doi: 10.1080/14712598.2025.2476030. Epub 2025 Mar 7. Expert Opin Biol Ther. 2025. PMID: 40035204 Review.
-
A Meta-Analysis of the Safety and Immunogenicity of Pharmacokinetic Similarity Studies and Comparative Clinical Studies.J Clin Pharmacol. 2025 Apr;65(4):499-507. doi: 10.1002/jcph.6165. Epub 2024 Nov 27. J Clin Pharmacol. 2025. PMID: 39604041
Cited by
-
A randomized, double-blind, single-dose study to assess bioequivalence of MB02 biosimilar after manufacturing iteration and reference bevacizumab.Pharmacol Res Perspect. 2023 Apr;11(2):e01070. doi: 10.1002/prp2.1070. Pharmacol Res Perspect. 2023. PMID: 36914963 Free PMC article. Clinical Trial.
-
Translation of Monoclonal Antibodies Pharmacokinetics from Animal to Human Using Physiologically Based Modeling in Open Systems Pharmacology (OSP) Suite: A Retrospective Analysis of Bevacizumab.Pharmaceutics. 2023 Aug 14;15(8):2129. doi: 10.3390/pharmaceutics15082129. Pharmaceutics. 2023. PMID: 37631343 Free PMC article.
-
Pooled analysis of three pharmacokinetic studies comparing biosimilar MB02 and reference bevacizumab.Pharmacol Res Perspect. 2023 Dec;11(6):e01139. doi: 10.1002/prp2.1139. Pharmacol Res Perspect. 2023. PMID: 37920875 Free PMC article.
-
Prediction of Monoclonal Antibody Pharmacokinetics in Pediatric Populations Using PBPK Modeling and Simulation.Pharmaceutics. 2025 Jul 5;17(7):884. doi: 10.3390/pharmaceutics17070884. Pharmaceutics. 2025. PMID: 40733093 Free PMC article.
-
Clinical Similarity of Biosimilars and Reference Drugs: A Comprehensive Review and New Hope for Public Health in a New Frontier.Curr Drug Res Rev. 2025;17(1):41-58. doi: 10.2174/0125899775246113231018080526. Curr Drug Res Rev. 2025. PMID: 37921214
References
-
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9‐22. - PubMed
-
- Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785‐1788. - PubMed
-
- European Medicines Agency (EMA) Guideline on similar biological medicinal product (Rev 1). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-si.... Accessed December 6, 2020.
-
- United States Federal Drug Administration (US FDA) Scientific considerations in demonstrating biosimilarity to a reference product. https://www.fda.gov/media/82647/download. Accessed December 6, 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

