Laser capture microdissection to study Bacillus cereus iron homeostasis gene expression during Galleria mellonella in vivo gut colonization
- PMID: 34374318
- PMCID: PMC8366545
- DOI: 10.1080/21505594.2021.1959790
Laser capture microdissection to study Bacillus cereus iron homeostasis gene expression during Galleria mellonella in vivo gut colonization
Abstract
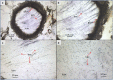
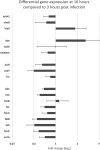
Bacillus cereus is a Gram-positive opportunistic pathogen closely related to the entomopathogen, Bacillus thuringiensis, both of which are involved in intestinal infections. Iron is an essential micronutrient for full growth and virulence of pathogens during infection. However, little is known about iron homeostasis during gut infection. Therefore, we aimed to assess the expression of B. cereus genes related to bacterial iron homeostasis, virulence and oxidative stress. The hypothesis is that the expression of such genes would vary between early and later stage colonization in correlation to gut cell damage. To perform the study, a germ-free Galleria mellonella model was set up in order to adapt the use of Laser-capture microdissection (LCM), to select precise areas in the gut lumen from frozen whole larval cryo-sections. Analyses were performed from alive larvae and the expression of targeted genes was assessed byspecific pre-amplification of mRNA followed by quantitative PCR. Firstly, the results reinforce the reliability of LCM, despite a low amount of bacterial RNA recovered. Secondly, bacterial genes involved in iron homeostasis are expressed in the lumen at both 3 and 16 hours post force-feeding. Thirdly, iron gene expression is slightly modulated during gut infection, and lastly, the mRNA of G. mellonella encoding for ferritin and transferrin iron storage and transport are recovered too. Therefore, iron homeostasis should play a role in B. cereus gut colonization. Furthermore, we demonstrate for the first time the value of using LCM for specific in situ gene expression analysis of extracellular bacteria in a whole animal.
Keywords: Laser-capture microdissection; bacillus cereus; colonization; galleria mellonella; gene expression; histology; in situ qPCR; insect model; intestinal infection; iron.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Andrews SC, Robinson AK, Rodríguez-Quiñones F.. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27(2–3):215–237. - PubMed
-
- Touati D.MINIREVIEW iron and oxidative stress in bacteria 1. Arch Biochem Biophys. 2000;373(1):1–6. - PubMed
-
- Nairz M, Schroll A, Sonnweber T, et al. The struggle for iron - a metal at the host-pathogen interface. Cell Microbiol. 2010;12(12):1691–1702. - PubMed
-
- Nobles CL, Maresso AW. The theft of host heme by Gram-positive pathogenic bacteria. Metallomics. 2011;3(8):788–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
