Tumour cell CD99 regulates transendothelial migration via CDC42 and actin remodelling
- PMID: 34374417
- PMCID: PMC8403985
- DOI: 10.1242/jcs.240135
Tumour cell CD99 regulates transendothelial migration via CDC42 and actin remodelling
Abstract
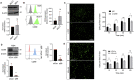
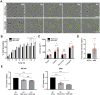
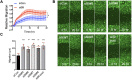
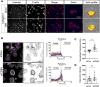
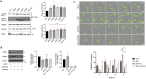
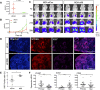
Metastasis requires tumour cells to cross endothelial cell (EC) barriers using pathways similar to those used by leucocytes during inflammation. Cell surface CD99 is expressed by healthy leucocytes and ECs, and participates in inflammatory transendothelial migration (TEM). Tumour cells also express CD99, and we have analysed its role in tumour progression and cancer cell TEM. Tumour cell CD99 was required for adhesion to ECs but inhibited invasion of the endothelial barrier and migratory activity. Furthermore, CD99 depletion in tumour cells caused redistribution of the actin cytoskeleton and increased activity of the Rho GTPase CDC42, known for its role in actin remodelling and cell migration. In a xenograft model of breast cancer, tumour cell CD99 expression inhibited metastatic progression, and patient samples showed reduced expression of the CD99 gene in brain metastases compared to matched primary breast tumours. We conclude that CD99 negatively regulates CDC42 and cell migration. However, CD99 has both pro- and anti-tumour activity, and our data suggest that this results in part from its functional linkage to CDC42 and the diverse signalling pathways downstream of this Rho GTPase. This article has an associated First Person interview with the first author of the paper.
Keywords: Actin cytoskeleton; Breast cancer; CD99; CDC42; Metastasis; Transendothelial migration.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
Similar articles
-
Structure and role of WASP and WAVE in Rho GTPase signalling in cancer.Cancer Genomics Proteomics. 2014 May-Jun;11(3):155-65. Cancer Genomics Proteomics. 2014. PMID: 24969695 Review.
-
Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases.Oncogene. 2020 Apr;39(18):3666-3679. doi: 10.1038/s41388-020-1243-2. Epub 2020 Mar 5. Oncogene. 2020. PMID: 32139877 Free PMC article.
-
MMP-9 affects gene expression in chronic lymphocytic leukemia revealing CD99 as an MMP-9 target and a novel partner in malignant cell migration/arrest.Oncogene. 2019 Jun;38(23):4605-4619. doi: 10.1038/s41388-019-0744-3. Epub 2019 Feb 13. Oncogene. 2019. PMID: 30760844
-
Roles of Rho GTPases in leucocyte and leukaemia cell transendothelial migration.Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130013. doi: 10.1098/rstb.2013.0013. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062583 Free PMC article. Review.
-
BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study.Mol Cancer. 2011 Sep 23;10:118. doi: 10.1186/1476-4598-10-118. Mol Cancer. 2011. PMID: 21943101 Free PMC article.
Cited by
-
Fucoxanthin prevents breast cancer metastasis by interrupting circulating tumor cells adhesion and transendothelial migration.Front Pharmacol. 2022 Sep 9;13:960375. doi: 10.3389/fphar.2022.960375. eCollection 2022. Front Pharmacol. 2022. PMID: 36160416 Free PMC article.
-
Decoding human in vitro terminal erythropoiesis originating from umbilical cord blood mononuclear cells and pluripotent stem cells.Cell Prolif. 2024 Jul;57(7):e13614. doi: 10.1111/cpr.13614. Epub 2024 Mar 18. Cell Prolif. 2024. PMID: 38499435 Free PMC article.
-
In Vitro Human Cancer Models for Biomedical Applications.Cancers (Basel). 2022 May 3;14(9):2284. doi: 10.3390/cancers14092284. Cancers (Basel). 2022. PMID: 35565413 Free PMC article. Review.
-
The pan-cancer landscape of crosstalk between leukocyte transendothelial migration-related genes and tumor microenvironment relevant to prognosis and immunotherapy response.Transl Cancer Res. 2024 Oct 31;13(10):5247-5264. doi: 10.21037/tcr-24-556. Epub 2024 Oct 25. Transl Cancer Res. 2024. PMID: 39525018 Free PMC article.
-
Pathological discrimination between luteinized thecoma associated with sclerosing peritonitis and thecoma.Medicine (Baltimore). 2023 Jun 9;102(23):e33911. doi: 10.1097/MD.0000000000033911. Medicine (Baltimore). 2023. PMID: 37335673 Free PMC article.
References
-
- Al Absi, A., Wurzer, H., Guerin, C., Hoffmann, C., Moreau, F., Mao, X., Brown-Clay, J., Petrolli, R., Casellas, C. P., Dieterle, M.et al. (2018). Actin cytoskeleton remodeling drives breast cancer cell escape from natural killer-mediated cytotoxicity. Cancer Res. 78, 5631-5643. 10.1158/0008-5472.CAN-18-0441 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

