Global Experience With Rotavirus Vaccines
- PMID: 34374426
- PMCID: PMC8687052
- DOI: 10.1093/infdis/jiab399
Global Experience With Rotavirus Vaccines
Abstract
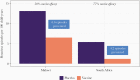
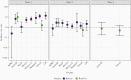
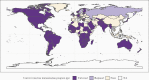
Rotavirus is a major cause of severe pediatric diarrhea worldwide. In 2006, 2 live, oral rotavirus vaccines, Rotarix and RotaTeq, were licensed for use in infants and were rapidly adopted in many high- and middle-income settings where efficacy had been demonstrated in clinical trials. Following completion of successful trials in low-income settings, the World Health Organization (WHO) recommended rotavirus vaccination for all infants globally in 2009. In 2018, 2 new rotavirus vaccines, Rotasiil and Rotavac, were prequalified by WHO, expanding global availability. As of March 2021, rotavirus vaccines have been introduced nationally in 106 countries. Since, Rotavirus vaccines have demonstrated effectiveness against severe disease and mortality, even among age groups in eligible for vaccination. Cross-genotypic protection has been demonstrated, and the favorable benefit-risk profile of these vaccines continues to be confirmed. Ongoing research seeks to better understand reasons for the geographic disparities in effectiveness observed, in order to optimize vaccine strategies worldwide.
Keywords: acute gastroenteritis; pediatric gastroenteritis; rotavirus; rotavirus vaccines; vaccine effectiveness; vaccine-preventable disease.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Epidemiology of rotavirus gastroenteritis and need of high rotavirus vaccine coverage with early completion of vaccination schedule for protection against rotavirus diarrhea in India: A narrative review.Indian J Public Health. 2019 Jul-Sep;63(3):243-250. doi: 10.4103/ijph.IJPH_307_18. Indian J Public Health. 2019. PMID: 31552856 Review.
-
The Rotavirus Vaccine Story: From Discovery to the Eventual Control of Rotavirus Disease.J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S331-S342. doi: 10.1093/infdis/jiaa598. J Infect Dis. 2021. PMID: 34590142 Free PMC article.
-
A randomized, open-labelled, non-inferiority phase 4 clinical trial to evaluate the immunogenicity and safety of the live, attenuated, oral rotavirus vaccine, ROTAVAC® in comparison with a licensed rotavirus vaccine in healthy infants.Vaccine. 2019 Jul 18;37(31):4407-4413. doi: 10.1016/j.vaccine.2019.05.069. Epub 2019 Jun 6. Vaccine. 2019. PMID: 31178377 Clinical Trial.
-
Rotavirus vaccines: an overview.Clin Microbiol Rev. 2008 Jan;21(1):198-208. doi: 10.1128/CMR.00029-07. Clin Microbiol Rev. 2008. PMID: 18202442 Free PMC article. Review.
-
Prevention of childhood rotavirus disease through the use of Rotarix and RotaTeq vaccines.Expert Opin Biol Ther. 2007 Dec;7(12):1881-92. doi: 10.1517/14712598.7.12.1881. Expert Opin Biol Ther. 2007. PMID: 18034653 Review.
Cited by
-
Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine.Vaccines (Basel). 2022 Sep 21;10(10):1585. doi: 10.3390/vaccines10101585. Vaccines (Basel). 2022. PMID: 36298449 Free PMC article.
-
Global Infection Rate of Rotavirus C during 1980-2022 and Analysis of Critical Factors in the Host Range Restriction of Virus VP4.Viruses. 2022 Dec 19;14(12):2826. doi: 10.3390/v14122826. Viruses. 2022. PMID: 36560830 Free PMC article.
-
Healthcare-Associated Gastroenteritis: Outbreak Report and Systematic Review of the Literature.J Pediatric Infect Dis Soc. 2025 Apr 8;14(4):piaf019. doi: 10.1093/jpids/piaf019. J Pediatric Infect Dis Soc. 2025. PMID: 40036241
-
Phylogeography of Rotavirus G8P[8] Detected in Argentina: Evidence of Transpacific Dissemination.Viruses. 2022 Oct 9;14(10):2223. doi: 10.3390/v14102223. Viruses. 2022. PMID: 36298778 Free PMC article.
-
Genotypic investigation of a rotavirus cluster at a quaternary-care pediatric hospital.Infect Control Hosp Epidemiol. 2023 Oct;44(10):1680-1682. doi: 10.1017/ice.2022.317. Epub 2023 Jan 24. Infect Control Hosp Epidemiol. 2023. PMID: 36691772 Free PMC article.
References
-
- Kapikian AZ, Shope RE. Rotaviruses, reoviruses, coltiviruses, and orbiviruses. In: Baron S, ed. Medical microbiology. Galveston, TX: University of Texas Medical Branch at Galveston, 1996. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

