Recurrent Vulvovaginal Candidiasis: a Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus
- PMID: 34374560
- PMCID: PMC8407231
- DOI: 10.1128/mSystems.00622-21
Recurrent Vulvovaginal Candidiasis: a Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus
Abstract
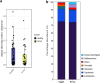
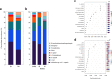
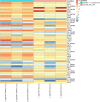
Despite the strikingly high worldwide prevalence of vulvovaginal candidiasis (VVC), treatment options for recurrent VVC (RVVC) remain limited, with many women experiencing failed clinical treatment with frontline azoles. Further, the cause of onset and recurrence of disease is largely unknown, with few studies identifying potential mechanisms of treatment failure. This study aimed to assess a panel of clinical samples from healthy women and those with RVVC to investigate the influence of Candida, the vaginal microbiome, and how their interaction influences disease pathology. 16S rRNA sequencing characterized disease by a reduction in specific health-associated Lactobacillus species, such as Lactobacillus crispatus, coupled with an increase in Lactobacillus iners. In vitro analysis showed that Candida albicans clinical isolates are capable of heterogeneous biofilm formation, and we found the presence of hyphae and C. albicans aggregates in vaginal lavage fluid. Additionally, the ability of Lactobacillus to inhibit C. albicans biofilm formation and biofilm-related gene expression was demonstrated. Using RNA sequencing technology, we were able to identify a possible mechanism by which L. crispatus may contribute to re-establishing a healthy vaginal environment through amino acid acquisition from C. albicans. This study highlights the potential formation and impact of Candida biofilms in RVVC. Additionally, it suggests that RVVC is not entirely due to an arbitrary switch in C. albicans from commensal to pathogen and that understanding interactions between this yeast and vaginal Lactobacillus species may be crucial to elucidating the cause of RVVC and developing appropriate therapies. IMPORTANCE RVVC is a significant burden, both economically and for women's health, but its prevalence is poorly documented globally due to the levels of self-treatment. Identifying triggers for development and recurrence of VVC and the pathogenesis of the microbes involved could considerably improve prevention and treatment options for women with recurrent, azole-resistant cases. This study therefore aimed to examine the interkingdom dynamics from healthy women and those with RVVC using next-generation sequencing techniques and to further investigate the molecular interactions between C. albicans and L. crispatus in a relevant biofilm coculture system.
Keywords: Candida; Lactobacillus; antifungal resistance; biofilm; clinical; interkingdom; microbiome; vulvovaginal candidiasis.
Figures






References
-
- Pizzorno JE, Murray MT, Joiner-Bey H. 2016. The clinician's handbook of natural medicine, 3rd ed, p 992. Elsevier. St. Louis, MO.
-
- Aballea S, Guelfucci F, Wagner J, Khemiri A, Dietz J-P, Sobel J, Toumi M. 2013. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis (RVVC) in Europe and the USA. Health Qual Life Outcomes 11:169. doi:10.1186/1477-7525-11-169. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
