Interstitial Lung Abnormalities: State of the Art
- PMID: 34374589
- PMCID: PMC8487219
- DOI: 10.1148/radiol.2021204367
Interstitial Lung Abnormalities: State of the Art
Abstract
The clinical importance of interstitial lung abnormality (ILA) is increasingly recognized. In July 2020, the Fleischner Society published a position paper about ILA. The purposes of this article are to summarize the definition, existing evidence, clinical management, and unresolved issues for ILA from a radiologic standpoint and to provide a practical guide for radiologists. ILA is a common incidental finding at CT and is often progressive and associated with worsened clinical outcomes. The hazard ratios for mortality range from 1.3 to 2.7 in large cohorts. Risk factors for ILA include age, smoking status, other inhalational exposures, and genetic factors (eg, gene encoding mucin 5B variant). Radiologists should systematically record the presence, morphologic characteristics, distribution, and subcategories of ILA (ie, nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic), as these are informative for predicting progression and mortality. Clinically significant interstitial lung disease should not be considered ILA. Individuals with ILA are triaged into higher- and lower-risk groups depending on their risk factors for progression, and systematic follow-up, including CT, should be considered for the higher-risk group. Artificial intelligence-based automated analysis for ILA may be helpful, but further validation and improvement are needed. Radiologists have a central role in clinical management and research on ILA.
© RSNA, 2021.
Conflict of interest statement
Figures

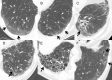
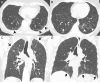
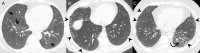

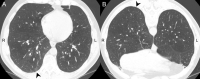
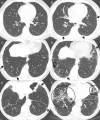
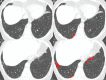
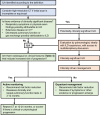
References
-
- Hatabu H , Hunninghake GM , Lynch DA . Interstitial lung abnormality: Recognition and perspectives . Radiology 2019. ; 291 ( 1 ): 1 – 3 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

