The Differential Metabolomes in Cumulus and Mural Granulosa Cells from Human Preovulatory Follicles
- PMID: 34374964
- PMCID: PMC8907092
- DOI: 10.1007/s43032-021-00691-3
The Differential Metabolomes in Cumulus and Mural Granulosa Cells from Human Preovulatory Follicles
Abstract
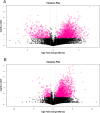
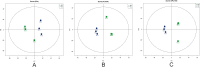
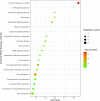
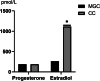
This study evaluated the differences in metabolites between cumulus cells (CCs) and mural granulosa cells (MGCs) from human preovulatory follicles to understand the mechanism of oocyte maturation involving CCs and MGCs. CCs and MGCs were collected from women who were undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatment. The differences in morphology were determined by immunofluorescence. The metabolomics of CCs and MGCs was measured by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) followed by quantitative polymerase chain reaction (qPCR) and western blot analysis to further confirm the genes and proteins involved in oocyte maturation. CCs and MGCs were cultured for 48 h in vitro, and the medium was collected for detection of hormone levels. There were minor morphological differences between CCs and MGCs. LC-MS/MS analysis showed that there were differences in 101 metabolites between CCs and MGCs: 7 metabolites were upregulated in CCs, and 94 metabolites were upregulated in MGCs. The metabolites related to cholesterol transport and estradiol production were enriched in CCs, while metabolites related to antiapoptosis were enriched in MGCs. The expression of genes and proteins involved in cholesterol transport (ABCA1, LDLR, and SCARB1) and estradiol production (SULT2B1 and CYP19A1) was significantly higher in CCs, and the expression of genes and proteins involved in antiapoptosis (CRLS1, LPCAT3, and PLA2G4A) was significantly higher in MGCs. The level of estrogen in CCs was significantly higher than that in MGCs, while the progesterone level showed no significant differences. There are differences between the metabolomes of CCs and MGCs. These differences may be involved in the regulation of oocyte maturation.
Keywords: Cumulus cells; Follicle; Human; Metabolomes; Mural granulosa cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Dysregulated sphingolipid metabolism and autophagy in granulosa cells of women with endometriosis.Front Endocrinol (Lausanne). 2022 Aug 3;13:906570. doi: 10.3389/fendo.2022.906570. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35992117 Free PMC article.
-
Change in the ability of bovine granulosa cells to elongate transzonal projections and their transcriptome changes during follicle development.J Reprod Dev. 2024 Dec 13;70(6):362-371. doi: 10.1262/jrd.2024-016. Epub 2024 Oct 14. J Reprod Dev. 2024. PMID: 39401905 Free PMC article.
-
Bone morphogenetic protein 6 expression in cumulus cells is negatively associated with oocyte maturation.Hum Fertil (Camb). 2021 Oct;24(4):290-297. doi: 10.1080/14647273.2019.1660003. Epub 2019 Sep 9. Hum Fertil (Camb). 2021. PMID: 31495245
-
The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization.Cells. 2021 Sep 2;10(9):2292. doi: 10.3390/cells10092292. Cells. 2021. PMID: 34571941 Free PMC article. Review.
-
The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome.Mol Hum Reprod. 2010 Oct;16(10):715-25. doi: 10.1093/molehr/gaq031. Epub 2010 Apr 29. Mol Hum Reprod. 2010. PMID: 20435609 Review.
Cited by
-
Role of EGFR expressed on the granulosa cells in the pathogenesis of polycystic ovarian syndrome.Front Endocrinol (Lausanne). 2022 Nov 11;13:971564. doi: 10.3389/fendo.2022.971564. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36440230 Free PMC article.
-
Primary ovarian insufficiency: update on clinical and genetic findings.Front Endocrinol (Lausanne). 2024 Sep 26;15:1464803. doi: 10.3389/fendo.2024.1464803. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39391877 Free PMC article. Review.
-
Unravelling the role of HAS2, GREM1, and PTGS2 gene expression in cumulus cells: implications for human oocyte development competency - a systematic review and integrated bioinformatic analysis.Front Endocrinol (Lausanne). 2024 Mar 8;15:1274376. doi: 10.3389/fendo.2024.1274376. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38524634 Free PMC article.
-
Role of pyroglutamic acid in cumulus cells of women with polycystic ovary syndrome.J Assist Reprod Genet. 2022 Dec;39(12):2737-2746. doi: 10.1007/s10815-022-02647-1. Epub 2022 Nov 2. J Assist Reprod Genet. 2022. PMID: 36322230 Free PMC article.
-
Combined Analysis of the Transcriptome, Proteome and Metabolome in Human Cryopreserved Sperm.World J Mens Health. 2024 Jul;42(3):610-619. doi: 10.5534/wjmh.230091. Epub 2024 Jan 2. World J Mens Health. 2024. PMID: 38164029 Free PMC article.
References
-
- Johnson ML, Redmer DA. Gap junctional intercellular communication of bovine granulosa and thecal cells from antral follicles: effects of luteinizing hormone and follicle-stimulating hormone. Endocrine. 2002;18(3):261–270. - PubMed
-
- Scott R III, M.Z.A.E.. Metabolism of the oocyte and the preimplantation embryo: implications for assisted reproduction. Curr Opin Obstet Gynecol. 2018;30(3):163–70. - PubMed
-
- Feuerstein P, Cadoret V. Gene expression in human CCs: one approach to oocyte competence. Hum Reprod. 2007;22:3069–3077. - PubMed
-
- Papler TB, Bokal EV. Specific gene expression differences in cumulus cells as potential biomarkers of pregnancy. Reprod BioMed Online. 2015;30(4):426–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

