The Current and Future Role of Radiation Therapy in the Era of CAR T-cell Salvage
- PMID: 34375124
- PMCID: PMC8553213
- DOI: 10.1259/bjr.20210098
The Current and Future Role of Radiation Therapy in the Era of CAR T-cell Salvage
Abstract
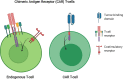
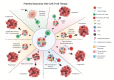
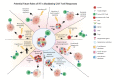
Radiation therapy has the potential to modulate the immune system in a variety of ways, and given the critical role of the immune system in cancer elimination, it is becoming increasingly important to understand how radiation can be strategically implemented in conjunction with approved immunotherapies to improve the cancer patient's chance of cure and/or quality of life. Current successful, approved cancer immunotherapies fall into two broad classes: antibodies and cellular therapies. Approved cellular therapies thus far consist of Chimeric Antigen Receptor (CAR) T-cells targeting CD19 for refractory non-Hodgkin lymphoma and relapsed or refractory acute lymphoblastic leukemia. Part of the ardor surrounding CAR T-cells stems from the fact that the survival curve of treated patients has a clear plateau, meaning that a number of patients with aggressive, disseminated disease who would have otherwise died rather rapidly appear to now be cured, commonly after just one dose. Despite an encouraging number of these durable remissions, the majority do still relapse. In this review, we discuss the potential for strategically utilizing radiation to further improve CAR T-cell patient outcomes. Given that there are currently over 750 cellular therapies in development, half of which are now in clinical trial, CAR T-cell usage will inevitably expand; as the field grows in importance and effectiveness, radiation oncology has the opportunity to coevolve symbiotically and steer these novel, exciting live therapies to new depths.
Figures
References
-
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-Term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20: 31–42. doi: 10.1016/S1470-2045(18)30864-7 - DOI - PMC - PubMed

